The BLM helicase is a new therapeutic target in multiple myeloma involved in replication stress survival and drug resistance
- PMID: 36569948
- PMCID: PMC9780552
- DOI: 10.3389/fimmu.2022.983181
The BLM helicase is a new therapeutic target in multiple myeloma involved in replication stress survival and drug resistance
Abstract
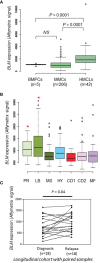
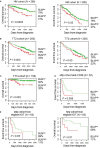
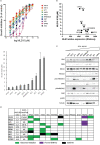
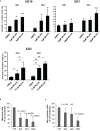
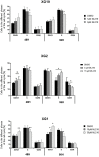
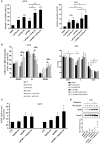
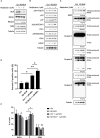
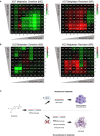
Multiple myeloma (MM) is a hematologic cancer characterized by accumulation of malignant plasma cells in the bone marrow. To date, no definitive cure exists for MM and resistance to current treatments is one of the major challenges of this disease. The DNA helicase BLM, whose depletion or mutation causes the cancer-prone Bloom's syndrome (BS), is a central factor of DNA damage repair by homologous recombination (HR) and genomic stability maintenance. Using independent cohorts of MM patients, we identified that high expression of BLM is associated with a poor outcome with a significant enrichment in replication stress signature. We provide evidence that chemical inhibition of BLM by the small molecule ML216 in HMCLs (human myeloma cell lines) leads to cell cycle arrest and increases apoptosis, likely by accumulation of DNA damage. BLM inhibition synergizes with the alkylating agent melphalan to efficiently inhibit growth and promote cell death in HMCLs. Moreover, ML216 treatment re-sensitizes melphalan-resistant cell lines to this conventional therapeutic agent. Altogether, these data suggest that inhibition of BLM in combination with DNA damaging agents could be of therapeutic interest in the treatment of MM, especially in those patients with high BLM expression and/or resistance to melphalan.
Keywords: BLM; DNA damage; drug resistance; multiple myeloma; replication stress.
Copyright © 2022 Ovejero, Viziteu, Dutrieux, Devin, Lin, Alaterre, Jourdan, Basbous, Requirand, Robert, de Boussac, Seckinger, Hose, Vincent, Herbaux, Constantinou, Pasero and Moreaux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Tacchetti P, Pantani L, Patriarca F, Petrucci MT, Zamagni E, Dozza L, et al. . Bortezomib, thalidomide, and dexamethasone followed by double autologous haematopoietic stem-cell transplantation for newly diagnosed multiple myeloma (GIMEMA-MMY-3006): Long-term follow-up analysis of a randomised phase 3, open-label study. Lancet Haematol (2020) 7:e861–73. doi: 10.1016/S2352-3026(20)30323-9 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

