Calixarene Functionalized Supramolecular Liquid Crystals and Their Diverse Applications
- PMID: 36570265
- PMCID: PMC9774433
- DOI: 10.1021/acsomega.2c04699
Calixarene Functionalized Supramolecular Liquid Crystals and Their Diverse Applications
Abstract
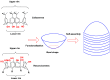
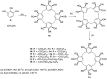
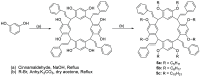
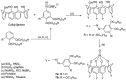
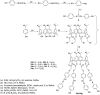
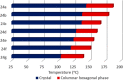
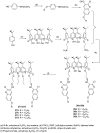
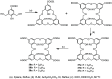
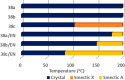
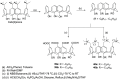
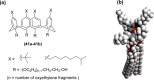
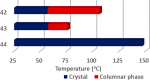
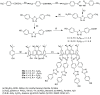
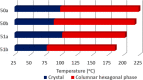
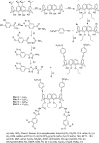
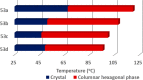
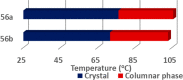
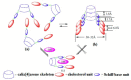
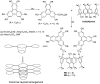
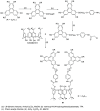
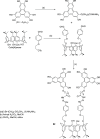
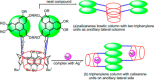
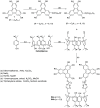
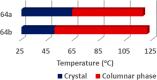
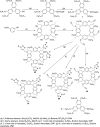
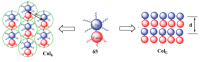
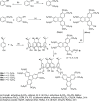
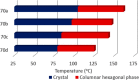
Liquid crystals are considered to be the fourth state of matter with an intermediate order and fluidity in comparison to solids and liquids. Calixarenes are among one of the most versatile families of building blocks for supramolecular chemistry due to their unique vaselike structure that can be chemically engineered to have different shapes and sizes. During the last few decades, calixarenes have drawn much attention in the field of supramolecular chemistry due to their diverse applications in the fields of ion and molecular recognition, ion-selective electrodes for catalysis, drug delivery, gelation, organic electronics and sensors, etc. Imbuing liquid crystallinity to the calixarene framework leads to functionalized calixarene derivatives with fluidity and order. Columnar self-assembly of such derivatives in particular enhance the charge migration along the column due to the 1D stacking due to the enhanced π-π overlap. Considering limited reports and reviews on this new class of calixarene based liquid crystals, a comprehensive account of the synthesis of calixarene liquid crystals along with their mesomorphic behavior and potential applications are presented in this review.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






























































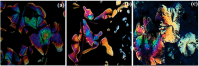



Similar articles
-
Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications.Acc Chem Res. 2014 Jul 15;47(7):1925-34. doi: 10.1021/ar500009g. Epub 2014 Mar 25. Acc Chem Res. 2014. PMID: 24666259 Review.
-
[Application of calixarene stationary phases to liquid chromatography].Se Pu. 2020 Apr 8;38(4):392-398. doi: 10.3724/SP.J.1123.2019.08027. Se Pu. 2020. PMID: 34213220 Chinese.
-
Perylene-Based Liquid Crystals as Materials for Organic Electronics Applications.Langmuir. 2019 Feb 19;35(7):2455-2479. doi: 10.1021/acs.langmuir.8b01081. Epub 2018 Jul 9. Langmuir. 2019. PMID: 29929366
-
Protein-Calixarene Complexation: From Recognition to Assembly.Acc Chem Res. 2022 Aug 2;55(15):2019-2032. doi: 10.1021/acs.accounts.2c00206. Epub 2022 Jun 6. Acc Chem Res. 2022. PMID: 35666543 Free PMC article.
-
Recent progress to construct calixarene-based polymers using covalent bonds: synthesis and applications.RSC Adv. 2020 Sep 3;10(54):32690-32722. doi: 10.1039/d0ra05707j. eCollection 2020 Sep 1. RSC Adv. 2020. PMID: 35516464 Free PMC article. Review.
Cited by
-
Ionic Liquid Crystals as Chromogenic Materials.Materials (Basel). 2024 Sep 17;17(18):4563. doi: 10.3390/ma17184563. Materials (Basel). 2024. PMID: 39336305 Free PMC article. Review.
-
Nano/Micro-Structural Supramolecular Biopolymers: Innovative Networks with the Boundless Potential in Sustainable Agriculture.Nanomicro Lett. 2024 Mar 8;16(1):147. doi: 10.1007/s40820-024-01348-x. Nanomicro Lett. 2024. PMID: 38457088 Free PMC article. Review.
References
-
- Collings P. J.Liquid Crystals: Nature’s Delicate Phase of Matter; Princeton University Press: 2002.
-
- Pal S. K.; Kumar S.. Liquid Crystal Dimers; Cambridge University Press: 2017.
-
- Kumar S.Chemistry of Discotic Liquid Crystals: From Monomers to Polymers; CRC Press: Boca Raton, FL, 2011.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

