Sequence Analysis and Structural Predictions of Lipid Transfer Bridges in the Repeating Beta Groove (RBG) Superfamily Reveal Past and Present Domain Variations Affecting Form, Function and Interactions of VPS13, ATG2, SHIP164, Hobbit and Tweek
- PMID: 36571082
- PMCID: PMC7613979
- DOI: 10.1177/25152564221134328
Sequence Analysis and Structural Predictions of Lipid Transfer Bridges in the Repeating Beta Groove (RBG) Superfamily Reveal Past and Present Domain Variations Affecting Form, Function and Interactions of VPS13, ATG2, SHIP164, Hobbit and Tweek
Abstract
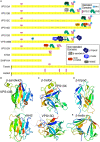
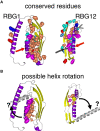
Lipid transfer between organelles requires proteins that shield the hydrophobic portions of lipids as they cross the cytoplasm. In the last decade a new structural form of lipid transfer protein (LTP) has been found: long hydrophobic grooves made of beta-sheet that bridge between organelles at membrane contact sites. Eukaryotes have five families of bridge-like LTPs: VPS13, ATG2, SHIP164, Hobbit and Tweek. These are unified into a single superfamily through their bridges being composed of just one domain, called the repeating beta groove (RBG) domain, which builds into rod shaped multimers with a hydrophobic-lined groove and hydrophilic exterior. Here, sequences and predicted structures of the RBG superfamily were analyzed in depth. Phylogenetics showed that the last eukaryotic common ancestor contained all five RBG proteins, with duplicated VPS13s. The current set of long RBG protein appears to have arisen in even earlier ancestors from shorter forms with 4 RBG domains. The extreme ends of most RBG proteins have amphipathic helices that might be an adaptation for direct or indirect bilayer interaction, although this has yet to be tested. The one exception to this is the C-terminus of SHIP164, which instead has a coiled-coil. Finally, the exterior surfaces of the RBG bridges are shown to have conserved residues along most of their length, indicating sites for partner interactions almost all of which are unknown. These findings can inform future cell biological and biochemical experiments.
Keywords: BLTP1; BLTP2; CLANS; ColabFold; Csf1; Fmp27; HHpred; UHRF1BP1L; UHRFBLP1L; Ypr117w.
Conflict of interest statement
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
