Screening of natural phenazine producers for electroactivity in bioelectrochemical systems
- PMID: 36571174
- PMCID: PMC9948232
- DOI: 10.1111/1751-7915.14199
Screening of natural phenazine producers for electroactivity in bioelectrochemical systems
Abstract
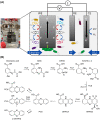
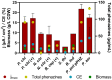
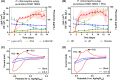
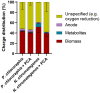
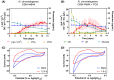
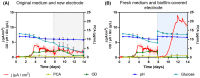
Mediated extracellular electron transfer (EET) might be a great vehicle to connect microbial bioprocesses with electrochemical control in stirred-tank bioreactors. However, mediated electron transfer to date is not only much less efficient but also much less studied than microbial direct electron transfer to an anode. For example, despite the widespread capacity of pseudomonads to produce phenazine natural products, only Pseudomonas aeruginosa has been studied for its use of phenazines in bioelectrochemical applications. To provide a deeper understanding of the ecological potential for the bioelectrochemical exploitation of phenazines, we here investigated the potential electroactivity of over 100 putative diverse native phenazine producers and the performance within bioelectrochemical systems. Five species from the genera Pseudomonas, Streptomyces, Nocardiopsis, Brevibacterium and Burkholderia were identified as new electroactive bacteria. Electron discharge to the anode and electric current production correlated with the phenazine synthesis of Pseudomonas chlororaphis subsp. aurantiaca. Phenazine-1-carboxylic acid was the dominant molecule with a concentration of 86.1 μg/ml mediating an anodic current of 15.1 μA/cm2 . On the other hand, Nocardiopsis chromatogenes used a wider range of phenazines at low concentrations and likely yet-unknown redox compounds to mediate EET, achieving an anodic current of 9.5 μA/cm2 . Elucidating the energetic and metabolic usage of phenazines in these and other species might contribute to improving electron discharge and respiration. In the long run, this may enhance oxygen-limited bioproduction of value-added compounds based on mediated EET mechanisms.
© 2022 The Authors. Microbial Biotechnology published by Applied Microbiology International and John Wiley & Sons Ltd.
Conflict of interest statement
There are no interests to declare.
Figures
References
-
- Bennur, T. , Ravi Kumar, A. , Zinjarde, S.S. & Javdekar, V. (2015) Nocardiopsis species: a potential source of bioactive compounds. Journal of Applied Microbiology, 120, 1–16. - PubMed
-
- Berger, C. & Rosenbaum, M.A. (2017) Spontaneous quorum sensing mutation modulates electroactivity of Pseudomonas aeruginosa PA14. Bioelectrochemistry, 117, 1–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

