Brain-derived tau: a novel blood-based biomarker for Alzheimer's disease-type neurodegeneration
- PMID: 36572122
- PMCID: PMC9976981
- DOI: 10.1093/brain/awac407
Brain-derived tau: a novel blood-based biomarker for Alzheimer's disease-type neurodegeneration
Erratum in
-
Correction to: Brain-derived tau: a novel blood-based biomarker for Alzheimer's disease-type neurodegeneration.Brain. 2023 Oct 3;146(10):e89-e90. doi: 10.1093/brain/awad208. Brain. 2023. PMID: 37365902 Free PMC article. No abstract available.
Abstract
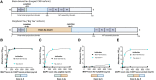
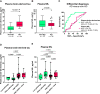
Blood-based biomarkers for amyloid beta and phosphorylated tau show good diagnostic accuracies and agreements with their corresponding CSF and neuroimaging biomarkers in the amyloid/tau/neurodegeneration [A/T/(N)] framework for Alzheimer's disease. However, the blood-based neurodegeneration marker neurofilament light is not specific to Alzheimer's disease while total-tau shows lack of correlation with CSF total-tau. Recent studies suggest that blood total-tau originates principally from peripheral, non-brain sources. We sought to address this challenge by generating an anti-tau antibody that selectively binds brain-derived tau and avoids the peripherally expressed 'big tau' isoform. We applied this antibody to develop an ultrasensitive blood-based assay for brain-derived tau, and validated it in five independent cohorts (n = 609) including a blood-to-autopsy cohort, CSF biomarker-classified cohorts and memory clinic cohorts. In paired samples, serum and CSF brain-derived tau were significantly correlated (rho = 0.85, P < 0.0001), while serum and CSF total-tau were not (rho = 0.23, P = 0.3364). Blood-based brain-derived tau showed equivalent diagnostic performance as CSF total-tau and CSF brain-derived tau to separate biomarker-positive Alzheimer's disease participants from biomarker-negative controls. Furthermore, plasma brain-derived tau accurately distinguished autopsy-confirmed Alzheimer's disease from other neurodegenerative diseases (area under the curve = 86.4%) while neurofilament light did not (area under the curve = 54.3%). These performances were independent of the presence of concomitant pathologies. Plasma brain-derived tau (rho = 0.52-0.67, P = 0.003), but not neurofilament light (rho = -0.14-0.17, P = 0.501), was associated with global and regional amyloid plaque and neurofibrillary tangle counts. These results were further verified in two memory clinic cohorts where serum brain-derived tau differentiated Alzheimer's disease from a range of other neurodegenerative disorders, including frontotemporal lobar degeneration and atypical parkinsonian disorders (area under the curve up to 99.6%). Notably, plasma/serum brain-derived tau correlated with neurofilament light only in Alzheimer's disease but not in the other neurodegenerative diseases. Across cohorts, plasma/serum brain-derived tau was associated with CSF and plasma AT(N) biomarkers and cognitive function. Brain-derived tau is a new blood-based biomarker that outperforms plasma total-tau and, unlike neurofilament light, shows specificity to Alzheimer's disease-type neurodegeneration. Thus, brain-derived tau demonstrates potential to complete the AT(N) scheme in blood, and will be useful to evaluate Alzheimer's disease-dependent neurodegenerative processes for clinical and research purposes.
Keywords: Alzheimer’s disease; neurodegenerative disease; neurofilament light; plasma brain-derived-tau; total-tau.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
M.T. and P.H. are employees of Bioventix Plc. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, and has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche. K.B. has served as a consultant or at advisory boards for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics and Siemens Healthineers. H.Z. and K.B. are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. The other authors declare no competing interests.
Figures



References
-
- Alzheimer’s Association . 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16:391–460.
-
- Ashton NJ, Hye A, Rajkumar AP, et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat Rev Neurol. 2020;16:265–284. - PubMed

