Regulatory role of endoplasmic reticulum resident chaperone protein ERp29 in anti-murine β-coronavirus host cell response
- PMID: 36572185
- PMCID: PMC9788854
- DOI: 10.1016/j.jbc.2022.102836
Regulatory role of endoplasmic reticulum resident chaperone protein ERp29 in anti-murine β-coronavirus host cell response
Abstract
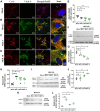
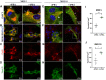
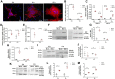
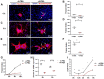
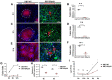
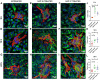
Gap junctional intercellular communication (GJIC) involving astrocytes is important for proper CNS homeostasis. As determined in our previous studies, trafficking of the predominant astrocyte GJ protein, Connexin43 (Cx43), is disrupted in response to infection with a neurotropic murine β-coronavirus (MHV-A59). However, how host factors are involved in Cx43 trafficking and the infection response is not clear. Here, we show that Cx43 retention due to MHV-A59 infection was associated with increased ER stress and reduced expression of chaperone protein ERp29. Treatment of MHV-A59-infected astrocytes with the chemical chaperone 4-sodium phenylbutyrate increased ERp29 expression, rescued Cx43 transport to the cell surface, increased GJIC, and reduced ER stress. We obtained similar results using an astrocytoma cell line (delayed brain tumor) upon MHV-A59 infection. Critically, delayed brain tumor cells transfected to express exogenous ERp29 were less susceptible to MHV-A59 infection and showed increased Cx43-mediated GJIC. Treatment with Cx43 mimetic peptides inhibited GJIC and increased viral susceptibility, demonstrating a role for intercellular communication in reducing MHV-A59 infectivity. Taken together, these results support a therapeutically targetable ERp29-dependent mechanism where β-coronavirus infectivity is modulated by reducing ER stress and rescuing Cx43 trafficking and function.
Keywords: Connexin43; ER stress; ERp29; MHV-A59; astrocytes; gap junctional intercellular communication (GJIC); murine coronavirus (mCoV); β-coronavirus infection.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest with the contents of the article.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

