3D collagen migration patterns reveal a SMAD3-dependent and TGF-β1-independent mechanism of recruitment for tumour-associated fibroblasts in lung adenocarcinoma
- PMID: 36572730
- PMCID: PMC10006167
- DOI: 10.1038/s41416-022-02093-x
3D collagen migration patterns reveal a SMAD3-dependent and TGF-β1-independent mechanism of recruitment for tumour-associated fibroblasts in lung adenocarcinoma
Abstract
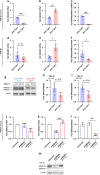
Background: The TGF-β1 transcription factor SMAD3 is epigenetically repressed in tumour-associated fibroblasts (TAFs) from lung squamous cell carcinoma (SCC) but not adenocarcinoma (ADC) patients, which elicits a compensatory increase in SMAD2 that renders SCC-TAFs less fibrotic. Here we examined the effects of altered SMAD2/3 in fibroblast migration and its impact on the desmoplastic stroma formation in lung cancer.
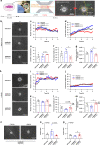
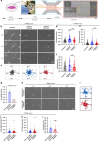
Methods: We used a microfluidic device to examine descriptors of early protrusions and subsequent migration in 3D collagen gels upon knocking down SMAD2 or SMAD3 by shRNA in control fibroblasts and TAFs.
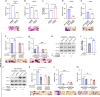
Results: High SMAD3 conditions as in shSMAD2 fibroblasts and ADC-TAFs exhibited a migratory advantage in terms of protrusions (fewer and longer) and migration (faster and more directional) selectively without TGF-β1 along with Erk1/2 hyperactivation. This enhanced migration was abrogated by TGF-β1 as well as low glucose medium and the MEK inhibitor Trametinib. In contrast, high SMAD2 fibroblasts were poorly responsive to TGF-β1, high glucose and Trametinib, exhibiting impaired migration in all conditions.
Conclusions: The basal migration advantage of high SMAD3 fibroblasts provides a straightforward mechanism underlying the larger accumulation of TAFs previously reported in ADC compared to SCC. Moreover, our results encourage using MEK inhibitors in ADC-TAFs but not SCC-TAFs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- FPU17/03867/Ministerio de Economía, Industria y Competitividad, Gobierno de España (Ministerio de Economía, Industria y Competitividad)
- RTI2018-094494-B-C21/Ministerio de Economía, Industria y Competitividad, Gobierno de España (Ministerio de Economía, Industria y Competitividad)
- PID2021-122409OB-C21/Ministerio de Economía, Industria y Competitividad, Gobierno de España (Ministerio de Economía, Industria y Competitividad)
- PI13/02368/Ministerio de Economía, Industria y Competitividad, Gobierno de España (Ministerio de Economía, Industria y Competitividad)
- SAF2016-79527-R/Ministerio de Economía, Industria y Competitividad, Gobierno de España (Ministerio de Economía, Industria y Competitividad)
- PID2019-110944RB-I00/Ministerio de Economía, Industria y Competitividad, Gobierno de España (Ministerio de Economía, Industria y Competitividad)
- PI14/01109/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI18/00920/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- Adg-101018587/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- SGR 661/Generalitat de Catalunya (Government of Catalonia)
- AECC B16-917/Fundación Científica Asociación Española Contra el Cáncer (Scientific Foundation, Spanish Association Against Cancer)
- SEPAR 951/2019/Sociedad Española de Neumología y Cirugía Torácica (SEPAR)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

