Use of Sequential Multiple Assignment Randomized Trials (SMARTs) in oncology: systematic review of published studies
- PMID: 36572731
- PMCID: PMC9792155
- DOI: 10.1038/s41416-022-02110-z
Use of Sequential Multiple Assignment Randomized Trials (SMARTs) in oncology: systematic review of published studies
Abstract
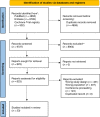
Sequential multiple assignments randomized trials (SMARTs) are a type of experimental design where patients may be randomised multiple times according to pre-specified decision rules. The present work investigates the state-of-the-art of SMART designs in oncology, focusing on the discrepancy between the available methodological approaches in the statistical literature and the procedures applied within cancer clinical trials. A systematic review was conducted, searching PubMed, Embase and CENTRAL for protocols or reports of results of SMART designs and registrations of SMART designs in clinical trial registries applied to solid tumour research. After title/abstract and full-text screening, 33 records were included. Fifteen were reports of trials' results, four were trials' protocols and fourteen were trials' registrations. The study design was defined as SMART by only one out of fifteen trial reports. Conversely, 13 of 18 study protocols and trial registrations defined the study design SMART. Furthermore, most of the records considered each stage separately in the analysis, without considering treatment regimens embedded in the trial. SMART designs in oncology are still limited. Study powering and analysis is mainly based on statistical approaches traditionally used in single-stage parallel trial designs. Formal reporting guidelines for SMART designs are needed.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources