Enzymatic and Microbial Electrochemistry: Approaches and Methods
- PMID: 36573075
- PMCID: PMC9783092
- DOI: 10.1021/acsmeasuresciau.2c00042
Enzymatic and Microbial Electrochemistry: Approaches and Methods
Abstract
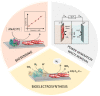
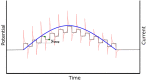
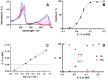
The coupling of enzymes and/or intact bacteria with electrodes has been vastly investigated due to the wide range of existing applications. These span from biomedical and biosensing to energy production purposes and bioelectrosynthesis, whether for theoretical research or pure applied industrial processes. Both enzymes and bacteria offer a potential biotechnological alternative to noble/rare metal-dependent catalytic processes. However, when developing these biohybrid electrochemical systems, it is of the utmost importance to investigate how the approaches utilized to couple biocatalysts and electrodes influence the resulting bioelectrocatalytic response. Accordingly, this tutorial review starts by recalling some basic principles and applications of bioelectrochemistry, presenting the electrode and/or biocatalyst modifications that facilitate the interaction between the biotic and abiotic components of bioelectrochemical systems. Focus is then directed toward the methods used to evaluate the effectiveness of enzyme/bacteria-electrode interaction and the insights that they provide. The basic concepts of electrochemical methods widely employed in enzymatic and microbial electrochemistry, such as amperometry and voltammetry, are initially presented to later focus on various complementary methods such as spectroelectrochemistry, fluorescence spectroscopy and microscopy, and surface analytical/characterization techniques such as quartz crystal microbalance and atomic force microscopy. The tutorial review is thus aimed at students and graduate students approaching the field of enzymatic and microbial electrochemistry, while also providing a critical and up-to-date reference for senior researchers working in the field.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Kano K.; et al. Voltammetric determination of acid dissociation constants of pyrroloquinoline quinone and its reduced form under acidic conditions. Bioelectrochemistry Bioenerg. 1990, 24, 193–201. 10.1016/0302-4598(90)85021-9. - DOI
Publication types
LinkOut - more resources
Full Text Sources
