Some Beneficial Effects of Inert Gases on Blood Oxidative Metabolism: In Vivo Study
- PMID: 36573196
- PMCID: PMC9789907
- DOI: 10.1155/2022/5857979
Some Beneficial Effects of Inert Gases on Blood Oxidative Metabolism: In Vivo Study
Abstract
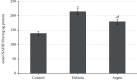
The aim of the study was to assess the effect of external use of inert gases (helium and argon) on the state of free radical processes in vivo. The experiment was performed on 30 male Wistar stock rats (age-3 months, weight-200-220 g.), randomly distributed into 3 equal groups. The first group of animals was intact (n = 10). The animals of the second and third groups were treated with argon and helium streams, respectively. Our research has allowed us to establish that the studied inert gases have a modulating effect on the state of oxidative metabolism of rat blood, and the nature of this effect is directly determined by the type of gas. The results of this study allowed us to establish the potential antioxidant effect of the helium stream, mainly realized due to the activation of the catalytic properties of the enzymatic link of the antioxidant system of rat blood plasma. At the same time, the revealed features of shifts in oxidative metabolism during treatment with argon flow include not only stimulation of the antioxidant system but also the pronounced induction of free radical oxidation. Thus, the conducted studies made it possible to verify the specificity of the response of the oxidative metabolism of blood plasma to the use of inert gases, depending on their type.
Copyright © 2022 Andrew Martusevich et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






References
-
- Featherstone R. M., Settle W. The pharmacology of the rare gases (helium, neon, argon, krypton, xenon) Actualites Pharmacologiques . 1974;27:69–86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

