The matricellular protein CCN3 supports lung endothelial homeostasis and function
- PMID: 36573684
- PMCID: PMC9925165
- DOI: 10.1152/ajplung.00248.2022
The matricellular protein CCN3 supports lung endothelial homeostasis and function
Abstract
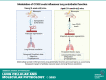
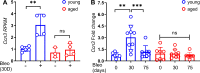
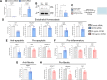
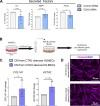
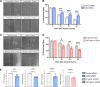
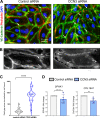
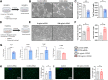
Aberrant vascular remodeling contributes to the progression of many aging-associated diseases, including idiopathic pulmonary fibrosis (IPF), where heterogeneous capillary density, endothelial transcriptional alterations, and increased vascular permeability correlate with poor disease outcomes. Thus, identifying disease-driving mechanisms in the pulmonary vasculature may be a promising strategy to limit IPF progression. Here, we identified Ccn3 as an endothelial-derived factor that is upregulated in resolving but not in persistent lung fibrosis in mice, and whose function is critical for vascular homeostasis and repair. Loss and gain of function experiments were carried out to test the role of CCN3 in lung microvascular endothelial function in vitro through RNAi and the addition of recombinant human CCN3 protein, respectively. Endothelial migration, permeability, proliferation, and in vitro angiogenesis were tested in cultured human lung microvascular endothelial cells (ECs). Loss of CCN3 in lung ECs resulted in transcriptional alterations along with impaired wound-healing responses, in vitro angiogenesis, barrier integrity as well as an increased profibrotic activity through paracrine signals, whereas the addition of recombinant CCN3 augmented endothelial function. Altogether, our results demonstrate that the matricellular protein CCN3 plays an important role in lung endothelial function and could serve as a promising therapeutic target to facilitate vascular repair and promote lung fibrosis resolution.
Keywords: CCN3; idiopathic pulmonary fibrosis; lung homeostasis; pulmonary fibrosis; pulmonary vasculature.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Decreased CCN3 in Systemic Sclerosis Endothelial Cells Contributes to Impaired Angiogenesis.J Invest Dermatol. 2020 Jul;140(7):1427-1434.e5. doi: 10.1016/j.jid.2019.11.026. Epub 2020 Jan 16. J Invest Dermatol. 2020. PMID: 31954725
-
Prostate cancer-derived CCN3 induces M2 macrophage infiltration and contributes to angiogenesis in prostate cancer microenvironment.Oncotarget. 2014 Mar 30;5(6):1595-608. doi: 10.18632/oncotarget.1570. Oncotarget. 2014. PMID: 24721786 Free PMC article.
-
Overexpression of CCN3 inhibits inflammation and progression of atherosclerosis in apolipoprotein E-deficient mice.PLoS One. 2014 Apr 10;9(4):e94912. doi: 10.1371/journal.pone.0094912. eCollection 2014. PLoS One. 2014. PMID: 24722330 Free PMC article.
-
The Emerging Roles of CCN3 Protein in Immune-Related Diseases.Mediators Inflamm. 2021 May 18;2021:5576059. doi: 10.1155/2021/5576059. eCollection 2021. Mediators Inflamm. 2021. PMID: 34393649 Free PMC article. Review.
-
Targeting endothelial cells: A novel strategy for pulmonary fibrosis treatment.Eur J Pharmacol. 2025 Jun 15;997:177472. doi: 10.1016/j.ejphar.2025.177472. Epub 2025 Mar 5. Eur J Pharmacol. 2025. PMID: 40054716 Review.
Cited by
-
The advance of CCN3 in fibrosis.J Cell Commun Signal. 2023 Dec;17(4):1219-1227. doi: 10.1007/s12079-023-00778-3. Epub 2023 Jun 28. J Cell Commun Signal. 2023. PMID: 37378812 Free PMC article. Review.
-
CCN proteins: opportunities for clinical studies-a personal perspective.J Cell Commun Signal. 2023 Jun;17(2):333-352. doi: 10.1007/s12079-023-00761-y. Epub 2023 May 17. J Cell Commun Signal. 2023. PMID: 37195381 Free PMC article. Review.
-
The vascular perspective on acute and chronic lung disease.J Clin Invest. 2023 Aug 15;133(16):e170502. doi: 10.1172/JCI170502. J Clin Invest. 2023. PMID: 37581311 Free PMC article. Review.
References
-
- Caporarello N, Lee J, Pham TX, Jones DL, Guan J, Link PA, Meridew JA, Marden G, Yamashita T, Osborne CA, Bhagwate AV, Huang SK, Nicosia RF, Tschumperlin DJ, Trojanowska M, Ligresti G. Dysfunctional ERG signaling drives pulmonary vascular aging and persistent fibrosis. Nat Commun 13: 4170, 2022. doi:10.1038/s41467-022-33197-w. - DOI - PMC - PubMed
-
- Caporarello N, Meridew JA, Aravamudhan A, Jones DL, Austin SA, Pham TX, Haak AJ, Moo Choi K, Tan Q, Haresi A, Huang SK, Katusic ZS, Tschumperlin DJ, Ligresti G. Vascular dysfunction in aged mice contributes to persistent lung fibrosis. Aging Cell 19: e13196, 2020. doi:10.1111/acel.13196. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

