Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors
- PMID: 36573850
- PMCID: PMC9908843
- DOI: 10.1002/anie.202217809
Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors
Abstract
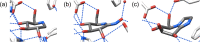
Substrate side chain conformation impacts reactivity during glycosylation and glycoside hydrolysis and is restricted by many glycosidases and glycosyltransferases during catalysis. We show that the side chains of gluco and manno iminosugars can be restricted to predominant conformations by strategic installation of a methyl group. Glycosidase inhibition studies reveal that iminosugars with the gauche,gauche side chain conformations are 6- to 10-fold more potent than isosteric compounds with the gauche,trans conformation; a manno-configured iminosugar with the gauche,gauche conformation is a 27-fold better inhibitor than 1-deoxymannojirimycin. The results are discussed in terms of the energetic benefits of preorganization, particularly when in synergy with favorable hydrophobic interactions. The demonstration that inhibitor side chain preorganization can favorably impact glycosidase inhibition paves the way for improved inhibitor design through conformational preorganization.
Keywords: Glycosidase; Iminosugar; Inhibitor; Methyl Group; Side Chain Conformation.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

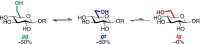




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

