ABX464 (Obefazimod) Upregulates miR-124 to Reduce Proinflammatory Markers in Inflammatory Bowel Diseases
- PMID: 36573890
- PMCID: PMC10132720
- DOI: 10.14309/ctg.0000000000000560
ABX464 (Obefazimod) Upregulates miR-124 to Reduce Proinflammatory Markers in Inflammatory Bowel Diseases
Abstract
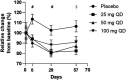
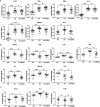
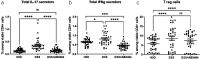
Advanced therapies have transformed the treatment of inflammatory bowel disease; however, many patients fail to respond, highlighting the need for therapies tailored to the underlying cell and molecular disease drivers. The first-in-class oral molecule ABX464 (obefazimod), which selectively upregulates miR-124, has demonstrated its ability to be a well-tolerated treatment with rapid and sustained efficacy in patients with ulcerative colitis (UC). Here, we provide evidence that ABX464 affects the immune system in vitro , in the murine model of inflammatory bowel disease, and in patients with UC. In vitro , ABX464 treatment upregulated miR-124 and led to decreases in proinflammatory cytokines including interleukin (IL) 17 and IL6, and in the chemokine CCL2. Consistently, miR-124 expression was upregulated in the rectal biopsies and blood samples of patients with UC, and a parallel reduction in Th17 cells and IL17a levels was observed in serum samples. In a mouse model of induced intestinal inflammation with dextran sulfate sodium, ABX464 reversed the increases in multiple proinflammatory cytokines in the colon and the upregulation of IL17a secretion in the mesenteric lymph nodes. By upregulating miR-124, ABX464 acts as "a physiological brake" of inflammation, which may explain the efficacy of ABX464 with a favorable tolerability and safety profile in patients with UC.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

