Photochemical Organocatalytic Functionalization of Pyridines via Pyridinyl Radicals
- PMID: 36574031
- PMCID: PMC9837848
- DOI: 10.1021/jacs.2c12466
Photochemical Organocatalytic Functionalization of Pyridines via Pyridinyl Radicals
Abstract
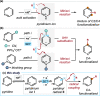
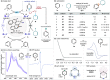
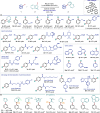
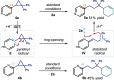
We report a photochemical method for the functionalization of pyridines with radicals derived from allylic C-H bonds. Overall, two substrates undergo C-H functionalization to form a new C(sp2)-C(sp3) bond. The chemistry harnesses the unique reactivity of pyridinyl radicals, generated upon single-electron reduction of pyridinium ions, which undergo effective coupling with allylic radicals. This novel mechanism enables distinct positional selectivity for pyridine functionalization that diverges from classical Minisci chemistry. Crucial was the identification of a dithiophosphoric acid that masters three catalytic tasks, sequentially acting as a Brønsted acid for pyridine protonation, a single electron transfer (SET) reductant for pyridinium ion reduction, and a hydrogen atom abstractor for the activation of allylic C(sp3)-H bonds. The resulting pyridinyl and allylic radicals then couple with high regioselectivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Joule J. A.; Mills K.. Heterocyclic Chemistry, 5th ed.; Wiley, 2010.
-
-
For a review, see:
- Proctor R. S. J.; Phipps R. J. Recent Advances in Minisci-Type Reactions. Angew. Chem., Int. Ed. 2019, 58, 13666–13699. and references therein10.1002/anie.201900977. - DOI - PubMed
-
For a seminal report, see:
- Minisci F.; Bernardi R.; Bertini F.; Galli R.; Perchinummo M. Nucleophilic character of alkyl radicals—VI: A new convenient selective alkylation of heteroaromatic bases. Tetrahedron 1971, 27, 3575–3580. 10.1016/S0040-4020(01)97768-3. - DOI
-
-
-
For a review, see:
- Tong S.; Li K.; Ouyang X.; Song R.; Li J. Recent advances in the radical-mediated decyanative alkylation of cyano(hetero)arene. Green Synth. Catal. 2021, 2, 145–155. 10.1016/j.gresc.2021.04.003. - DOI
-
For a seminal example, see:
- Caronna T.; Clerici A.; Coggiola D.; Morrocchi S. Photoreactions of 4-Cyanopyridine with Alkenols. Influence of the Medium on the Reaction Mechanism and Photoproducts Formation. Tetrahedron Lett. 1981, 22, 2115–2118. 10.1016/S0040-4039(01)93292-7. - DOI
-
For selected recent examples, see:
- McNally A.; Prier C. K.; MacMillan D. W. C. Discovery of an α-Amino C-H Arylation Reaction using the Strategy of Accelerated Serendipity. Science 2011, 334, 1114–1117. 10.1126/science.1213920. - DOI - PMC - PubMed
- Hoshikawa T.; Inoue M. Photoinduced Direct 4-Pyridination of C(sp3)–H Bonds. Chem. Sci. 2013, 4, 3118–3123. 10.1039/c3sc51080h. - DOI
-
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

