TRPM7 kinase is required for insulin production and compensatory islet responses during obesity
- PMID: 36574297
- PMCID: PMC9977431
- DOI: 10.1172/jci.insight.163397
TRPM7 kinase is required for insulin production and compensatory islet responses during obesity
Abstract
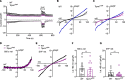
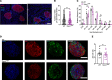
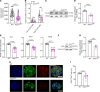
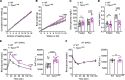
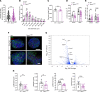
Most overweight individuals do not develop diabetes due to compensatory islet responses to restore glucose homeostasis. Therefore, regulatory pathways that promote β cell compensation are potential targets for treatment of diabetes. The transient receptor potential cation channel subfamily M member 7 protein (TRPM7), harboring a cation channel and a serine/threonine kinase, has been implicated in controlling cell growth and proliferation. Here, we report that selective deletion of Trpm7 in β cells disrupted insulin secretion and led to progressive glucose intolerance. We indicate that the diminished insulinotropic response in β cell-specific Trpm7-knockout mice was caused by decreased insulin production because of impaired enzymatic activity of this protein. Accordingly, high-fat-fed mice with a genetic loss of TRPM7 kinase activity displayed a marked glucose intolerance accompanied by hyperglycemia. These detrimental glucoregulatory effects were engendered by reduced compensatory β cell responses because of mitigated protein kinase B (AKT)/ERK signaling. Collectively, our data identify TRPM7 kinase as a potentially novel regulator of insulin synthesis, β cell dynamics, and glucose homeostasis under obesogenic diet.
Keywords: Beta cells; Cell Biology; Insulin; Ion channels.
Conflict of interest statement
Figures


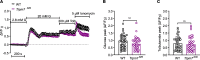
Similar articles
-
Mg2+ Supplementation Mitigates Metabolic Deficits Associated With TRPM7 Disruption.J Cell Physiol. 2025 Apr;240(4):e70042. doi: 10.1002/jcp.70042. J Cell Physiol. 2025. PMID: 40275767 Free PMC article.
-
TRPM7 is a crucial regulator of pancreatic endocrine development and high-fat-diet-induced β-cell proliferation.Development. 2021 Aug 15;148(16):dev194928. doi: 10.1242/dev.194928. Epub 2021 Aug 16. Development. 2021. PMID: 34345920 Free PMC article.
-
TRPM6 kinase activity regulates TRPM7 trafficking and inhibits cellular growth under hypomagnesic conditions.Cell Mol Life Sci. 2014 Dec;71(24):4853-67. doi: 10.1007/s00018-014-1647-7. Epub 2014 May 25. Cell Mol Life Sci. 2014. PMID: 24858416 Free PMC article.
-
Function and regulation of the channel-kinase TRPM7 in health and disease.Eur J Cell Biol. 2014 Oct;93(10-12):455-65. doi: 10.1016/j.ejcb.2014.07.001. Epub 2014 Jul 8. Eur J Cell Biol. 2014. PMID: 25073440 Review.
-
Mapping TRPM7 Function by NS8593.Int J Mol Sci. 2020 Sep 23;21(19):7017. doi: 10.3390/ijms21197017. Int J Mol Sci. 2020. PMID: 32977698 Free PMC article. Review.
Cited by
-
Inactivation of TRPM7 Kinase Targets AKT Signaling and Cyclooxygenase-2 Expression in Human CML Cells.Function (Oxf). 2023 Sep 15;4(6):zqad053. doi: 10.1093/function/zqad053. eCollection 2023. Function (Oxf). 2023. PMID: 37786778 Free PMC article.
-
Inter-Organellar Ca2+ Homeostasis in Plant and Animal Systems.Cells. 2025 Aug 6;14(15):1204. doi: 10.3390/cells14151204. Cells. 2025. PMID: 40801637 Free PMC article. Review.
-
Mg2+ Supplementation Mitigates Metabolic Deficits Associated With TRPM7 Disruption.J Cell Physiol. 2025 Apr;240(4):e70042. doi: 10.1002/jcp.70042. J Cell Physiol. 2025. PMID: 40275767 Free PMC article.
-
LncRNA SNHG8 regulates the migration and angiogenesis of pHUVECs induced by high glucose via the TRPM7/ERK1/2 signaling axis.Sci Rep. 2023 Dec 18;13(1):22485. doi: 10.1038/s41598-023-49779-7. Sci Rep. 2023. PMID: 38110485 Free PMC article.
-
Chronic Mg2+ Deficiency Does Not Impair Insulin Secretion in Mice.Cells. 2023 Jul 5;12(13):1790. doi: 10.3390/cells12131790. Cells. 2023. PMID: 37443824 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

