Carotenoids as potential inhibitors of TNFα in COVID-19 treatment
- PMID: 36574379
- PMCID: PMC9794061
- DOI: 10.1371/journal.pone.0276538
Carotenoids as potential inhibitors of TNFα in COVID-19 treatment
Abstract
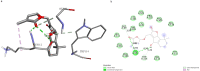
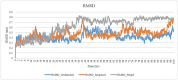
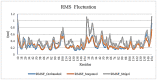
Tumor necrosis factor-alpha (TNF-α) is a multifunctional pro-inflammatory cytokine, responsible for autoimmune and inflammatory disorders. In COVID-19 patients, increased TNF-α concentration may provoke inflammatory cascade and induce the initiation of cytokine storm that may result in fatal pneumonia and acute respiratory distress syndrome (ADRS). Hence, TNFα is assumed to be a promising drug target against cytokine storm in COVID-19 patients. In the present study, we focused on finding novel small molecules that can directly block TNF-α-hTNFR1 (human TNF receptor 1) interaction. In this regards, TNF-α-inhibiting capacity of natural carotenoids was investigated in terms of blocking TNF-α-hTNFR1 interaction in COVID-19 patients with the help of a combination of in silico approaches, based on virtual screening, molecular docking, and molecular dynamics (MD) simulation. A total of 125 carotenoids were selected out of 1204 natural molecules, based on their pharmacokinetics properties and they all met Lipinski's rule of five. Among them, Sorgomol, Strigol and Orobanchol had the most favorable ΔG with the best ADME (absorption, distribution, metabolism, excretion) properties, and were selected for MD simulation studies, which explored the complex stability and the impact of ligands on protein conformation. Our results showed that Sorgomol formed the most hydrogen bonds, resulting in the highest binding energy with lowest RMSD and RMSF, which made it the most appropriate candidate as TNF-α inhibitor. In conclusion, the present study could serve to expand possibilities to develop new therapeutic small molecules against TNF-α.
Copyright: © 2022 Taghipour et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

