Kidney-resident innate-like memory γδ T cells control chronic Staphylococcus aureus infection of mice
- PMID: 36574651
- PMCID: PMC9910431
- DOI: 10.1073/pnas.2210490120
Kidney-resident innate-like memory γδ T cells control chronic Staphylococcus aureus infection of mice
Abstract
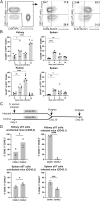
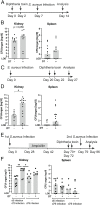
γδ T cells are involved in the control of Staphylococcus aureus infection, but their importance in protection compared to other T cells is unclear. We used a mouse model of systemic S. aureus infection associated with high bacterial load and persistence in the kidney. Infection caused fulminant accumulation of γδ T cells in the kidney. Renal γδ T cells acquired tissue residency and were maintained in high numbers during chronic infection. At day 7, up to 50% of renal γδ T cells produced IL-17A in situ and a large fraction of renal γδ T cells remained IL-17A+ during chronic infection. Controlled depletion revealed that γδ T cells restricted renal S. aureus replication in the acute infection and provided protection during chronic renal infection and upon reinfection. Our results demonstrate that kidney-resident γδ T cells are nonredundant in limiting local S. aureus growth during chronic infection and provide enhanced protection against reinfection.
Keywords: IL-17; Staphylococcus aureus; T cell memory; tissue residency; γδ T cells.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Kang C. I., et al. , Bloodstream infections in adult patients with cancer: Clinical features and pathogenic significance of Staphylococcus aureus bacteremia. Support Care Cancer. 20, 2371–2378 (2012). - PubMed
-
- Buvelot H., Posfay-Barbe K. M., Linder P., Schrenzel J., Krause K. H., Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. F.E.M.S. Microbiol. Rev. 41, 139–157 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

