Crystal structure of LGR ligand α2/β5 from Caenorhabditis elegans with implications for the evolution of glycoprotein hormones
- PMID: 36574673
- PMCID: PMC9910494
- DOI: 10.1073/pnas.2218630120
Crystal structure of LGR ligand α2/β5 from Caenorhabditis elegans with implications for the evolution of glycoprotein hormones
Abstract
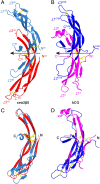
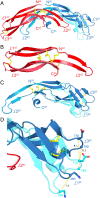
A family of leucine-rich-repeat-containing G-protein-coupled receptors (LGRs) mediate diverse physiological responses when complexed with their cognate ligands. LGRs are present in all metazoan animals. In humans, the LGR ligands include glycoprotein hormones (GPHs) chorionic gonadotropin (hCG), luteinizing hormone, follicle-stimulating hormone (hFSH), and thyroid-stimulating hormone (hTSH). These hormones are αβ heterodimers of cystine-knot protein chains. LGRs and their ligand chains have coevolved. Ancestral hormone homologs, present in both bilaterian animals and chordates, are identified as α2β5. We have used single-wavelength anomalous diffraction and molecular replacement to determine structures of the α2β5 hormone from Caenorhabditis elegans (Ceα2β5). Ceα2β5 is unglycosylated, as are many other α2β5 hormones. Both Hsα2β5, the human homolog of Ceα2β5, and hTSH activate the same receptor (hTSHR). Despite having little sequence similarity to vertebrate GPHs, apart from the cysteine patterns from core disulfide bridges, Ceα2β5 is generally similar in structure to these counterparts; however, its α2 and β5 subunits are more symmetric as compared with α and β of hCG and hFSH. This quasisymmetry suggests a hypothetical homodimeric antecedent of the α2β5 and αβ heterodimers. Known structures together with AlphaFold models from the sequences for other LGR ligands provide representatives for the molecular evolution of LGR ligands from early metazoans through the present-day GPHs. The experimental Ceα2β5 structure validates its AlphaFold model, and thus also that for Hsα2β5; and interfacial characteristics in a model for the Hsα2β5:hTSHR complex are similar to those found in an experimental hTSH:hTSHR structure.
Keywords: cystine-knot hormone (CKH); evolution; glycoprotein hormone (GPH); leucine-rich-repeat-containing G-protein-coupled receptor (LGR); thyrostimulin.
Conflict of interest statement
The authors have research support to disclose, NIH GM107462.
Figures




Similar articles
-
Glycoprotein hormones and their receptors emerged at the origin of metazoans.Genome Biol Evol. 2014 Jun 5;6(6):1466-79. doi: 10.1093/gbe/evu118. Genome Biol Evol. 2014. PMID: 24904013 Free PMC article.
-
Structural and evolutionary insights into the functioning of glycoprotein hormones and their receptors.Andrology. 2025 Jan 27. doi: 10.1111/andr.70001. Online ahead of print. Andrology. 2025. PMID: 39871527 Review.
-
Conservation of the heterodimeric glycoprotein hormone subunit family proteins and the LGR signaling system from nematodes to humans.Endocrine. 2005 Apr;26(3):267-76. doi: 10.1385/ENDO:26:3:267. Endocrine. 2005. PMID: 16034181
-
The nematode leucine-rich repeat-containing, G protein-coupled receptor (LGR) protein homologous to vertebrate gonadotropin and thyrotropin receptors is constitutively active in mammalian cells.Mol Endocrinol. 2000 Feb;14(2):272-84. doi: 10.1210/mend.14.2.0422. Mol Endocrinol. 2000. PMID: 10674399
-
Comparative structure analyses of cystine knot-containing molecules with eight aminoacyl ring including glycoprotein hormones (GPH) alpha and beta subunits and GPH-related A2 (GPA2) and B5 (GPB5) molecules.Reprod Biol Endocrinol. 2009 Aug 31;7:90. doi: 10.1186/1477-7827-7-90. Reprod Biol Endocrinol. 2009. PMID: 19715619 Free PMC article. Review.
Cited by
-
Facing the phase problem.IUCrJ. 2023 Sep 1;10(Pt 5):521-543. doi: 10.1107/S2052252523006449. IUCrJ. 2023. PMID: 37668214 Free PMC article.
-
Structure of an LGR dimer - an evolutionary predecessor of glycoprotein hormone receptors.bioRxiv [Preprint]. 2025 Jan 2:2024.12.31.630923. doi: 10.1101/2024.12.31.630923. bioRxiv. 2025. PMID: 39803540 Free PMC article. Preprint.
-
Ancestral glycoprotein hormone-receptor pathway controls growth in C. elegans.Front Endocrinol (Lausanne). 2023 Jun 20;14:1200407. doi: 10.3389/fendo.2023.1200407. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37409228 Free PMC article.
-
AlphaFold-guided molecular replacement for solving challenging crystal structures.Acta Crystallogr D Struct Biol. 2025 Jan 1;81(Pt 1):4-21. doi: 10.1107/S2059798324011999. Epub 2025 Jan 1. Acta Crystallogr D Struct Biol. 2025. PMID: 39711199
-
Evolutionary conserved peptide and glycoprotein hormone-like neuroendocrine systems in C. elegans.Mol Cell Endocrinol. 2024 Apr 15;584:112162. doi: 10.1016/j.mce.2024.112162. Epub 2024 Jan 28. Mol Cell Endocrinol. 2024. PMID: 38290646 Free PMC article.
References
-
- Pierce J. G., Parsons T. F., Glycoprotein hormones: Structure and function. Annu. Rev. Biochem. 50, 465–495 (1981). - PubMed
-
- Jiang X., Dias J. A., He X., Structural biology of glycoprotein hormones and their receptors: Insights to signaling. Mol. Cell Endocrinol. 382, 424–451 (2014). - PubMed
-
- Hsu S. Y., et al. , The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): Identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol. Endocrinol. 14, 1257–1271 (2000). - PubMed
-
- Ascoli M., Fanelli F., Segaloff D. L., The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 23, 141–174 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

