NADP(H)-dependent biocatalysis without adding NADP(H)
- PMID: 36574703
- PMCID: PMC9910440
- DOI: 10.1073/pnas.2214123120
NADP(H)-dependent biocatalysis without adding NADP(H)
Abstract
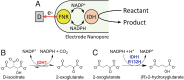
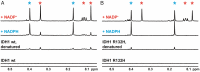
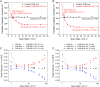
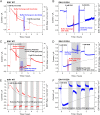
Isocitrate dehydrogenase 1 (IDH1) naturally copurifies and crystallizes in a resting state with a molecule of its exchangeable cofactor, NADP+/NADPH, bound in each monomer of the homodimer. We report electrochemical studies with IDH1 that exploit this property to reveal the massive advantage of nanoconfinement to increase the efficiency of multistep enzyme-catalyzed cascade reactions. When coloaded with ferredoxin NADP+ reductase in a nanoporous conducting indium tin oxide film, IDH1 carries out the complete electrochemical oxidation of 6 mM isocitrate (in 4mL) to 2-oxoglutarate (2OG), using only the NADP(H) that copurified with IDH1 and was carried into the electrode pores as cargo-the system remains active for days. The entrapped cofactor, now quantifiable by cyclic voltammetry, undergoes ~160,000 turnovers during the process. The results from a variety of electrocatalysis experiments imply that the local concentrations of the two nanoconfined enzymes lie around the millimolar range. The combination of crowding and entrapment results in a 102 to 103-fold increase in the efficiency of NADP(H) redox cycling. The ability of the method to drive cascade catalysis in either direction (oxidation or reduction) and remove and replace substrates was exploited to study redox-state dependent differences in cofactor binding between wild-type IDH1 and the cancer-linked R132H variant that catalyzes the "gain of function" reduction of 2OG to 2-hydroxyglutarate instead of isocitrate oxidation. The combined results demonstrate the power of nanoconfinement for facilitating multistep enzyme catalysis (in this case energized and verified electrochemically) and reveal insights into the dynamic role of nicotinamide cofactors as redox (hydride) carriers.
Keywords: NADPH; biocatalysis; electrocatalysis; isocitrate dehydrogenase; nanoconfinement.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Exploiting Electrode Nanoconfinement to Investigate the Catalytic Properties of Isocitrate Dehydrogenase (IDH1) and a Cancer-Associated Variant.J Phys Chem Lett. 2021 Jul 8;12(26):6095-6101. doi: 10.1021/acs.jpclett.1c01517. Epub 2021 Jun 25. J Phys Chem Lett. 2021. PMID: 34170697 Free PMC article.
-
From Protein Film Electrochemistry to Nanoconfined Enzyme Cascades and the Electrochemical Leaf.Chem Rev. 2023 May 10;123(9):5421-5458. doi: 10.1021/acs.chemrev.2c00397. Epub 2022 Dec 27. Chem Rev. 2023. PMID: 36573907 Free PMC article. Review.
-
A hydrogen bond network in the active site of Anabaena ferredoxin-NADP(+) reductase modulates its catalytic efficiency.Biochim Biophys Acta. 2014 Feb;1837(2):251-63. doi: 10.1016/j.bbabio.2013.10.010. Epub 2013 Nov 4. Biochim Biophys Acta. 2014. PMID: 24200908
-
High-resolution studies of hydride transfer in the ferredoxin:NADP+ reductase superfamily.FEBS J. 2017 Oct;284(19):3302-3319. doi: 10.1111/febs.14190. Epub 2017 Aug 29. FEBS J. 2017. PMID: 28783258 Free PMC article.
-
Isocitrate dehydrogenase variants in cancer - Cellular consequences and therapeutic opportunities.Curr Opin Chem Biol. 2020 Aug;57:122-134. doi: 10.1016/j.cbpa.2020.06.012. Epub 2020 Aug 8. Curr Opin Chem Biol. 2020. PMID: 32777735 Free PMC article. Review.
Cited by
-
Building Localized NADP(H) Recycling Circuits to Advance Enzyme Cascadetronics.Angew Chem Int Ed Engl. 2025 Mar 3;64(10):e202414176. doi: 10.1002/anie.202414176. Epub 2025 Feb 11. Angew Chem Int Ed Engl. 2025. PMID: 39876743 Free PMC article.
-
Electrochemical Nanoreactor Provides a Comprehensive View of Isocitrate Dehydrogenase Cancer-drug Kinetics.Angew Chem Int Ed Engl. 2023 Oct 16;62(42):e202309149. doi: 10.1002/anie.202309149. Epub 2023 Sep 12. Angew Chem Int Ed Engl. 2023. PMID: 37607127 Free PMC article.
-
Active site remodeling in tumor-relevant IDH1 mutants drives distinct kinetic features and potential resistance mechanisms.Nat Commun. 2024 May 6;15(1):3785. doi: 10.1038/s41467-024-48277-2. Nat Commun. 2024. PMID: 38710674 Free PMC article.
-
Active site remodeling in tumor-relevant IDH1 mutants drives distinct kinetic features and potential resistance mechanisms.bioRxiv [Preprint]. 2024 Jan 23:2024.01.10.574970. doi: 10.1101/2024.01.10.574970. bioRxiv. 2024. Update in: Nat Commun. 2024 May 6;15(1):3785. doi: 10.1038/s41467-024-48277-2. PMID: 38260668 Free PMC article. Updated. Preprint.
-
Extending protein-film electrochemistry across enzymology and biological inorganic chemistry to investigate, track and control the reactions of non-redox enzymes and spectroscopically silent metals.J Biol Inorg Chem. 2025 Apr;30(3):209-219. doi: 10.1007/s00775-025-02105-0. Epub 2025 Mar 1. J Biol Inorg Chem. 2025. PMID: 40025220 Free PMC article. Review.
References
-
- Vázquez-González M., Wang C., Willner I., Biocatalytic cascades operating on macromolecular scaffolds and in confined environments. Nat. Catal. 3, 256–273 (2020).
-
- Küchler A., Yoshimoto M., Luginbühl S., Mavelli F., Walde P., Enzymatic reactions in confined environments. Nat. Nanotechnol. 11, 409–420 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- C8717/A18245/CRUK_/Cancer Research UK/United Kingdom
- BB/P023797/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 28285/CRUK_/Cancer Research UK/United Kingdom
- 106244/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- BB/R506655/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

