A RGS7-CaMKII complex drives myocyte-intrinsic and myocyte-extrinsic mechanisms of chemotherapy-induced cardiotoxicity
- PMID: 36574707
- PMCID: PMC9910480
- DOI: 10.1073/pnas.2213537120
A RGS7-CaMKII complex drives myocyte-intrinsic and myocyte-extrinsic mechanisms of chemotherapy-induced cardiotoxicity
Abstract
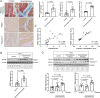
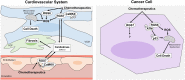
Dose-limiting cardiotoxicity remains a major limitation in the clinical use of cancer chemotherapeutics. Here, we describe a role for Regulator of G protein Signaling 7 (RGS7) in chemotherapy-dependent heart damage, the demonstration for a functional role of RGS7 outside of the nervous system and retina. Though expressed at low levels basally, we observed robust up-regulation of RGS7 in the human and murine myocardium following chemotherapy exposure. In ventricular cardiomyocytes (VCM), RGS7 forms a complex with Ca2+/calmodulin-dependent protein kinase (CaMKII) supported by key residues (K412 and P391) in the RGS domain of RGS7. In VCM treated with chemotherapeutic drugs, RGS7 facilitates CaMKII oxidation and phosphorylation and CaMKII-dependent oxidative stress, mitochondrial dysfunction, and apoptosis. Cardiac-specific RGS7 knockdown protected the heart against chemotherapy-dependent oxidative stress, fibrosis, and myocyte loss and improved left ventricular function in mice treated with doxorubicin. Conversely, RGS7 overexpression induced fibrosis, reactive oxygen species generation, and cell death in the murine myocardium that were mitigated following CaMKII inhibition. RGS7 also drives production and release of the cardiokine neuregulin-1, which facilitates paracrine communication between VCM and neighboring vascular endothelial cells (EC), a maladaptive mechanism contributing to VCM dysfunction in the failing heart. Importantly, while RGS7 was both necessary and sufficient to facilitate chemotherapy-dependent cytotoxicity in VCM, RGS7 is dispensable for the cancer-killing actions of these same drugs. These selective myocyte-intrinsic and myocyte-extrinsic actions of RGS7 in heart identify RGS7 as an attractive therapeutic target in the mitigation of chemotherapy-driven cardiotoxicity.
Keywords: RGS protein; cardiotoxicity; cell death; chemotherapy; oxidative stress.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
-
- Henriksen P. A., Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 104, 971–977 (2018). - PubMed
-
- Cardinale D., et al. , Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109, 2749–2754 (2004). - PubMed
-
- Lenihan D. J., et al. , The utility of point-of-care biomarkers to detect cardiotoxicity during anthracycline chemotherapy: A feasibility study. J. Card Fail 22, 433–438 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

