Itaconate promotes a wound resolving phenotype in pro-inflammatory macrophages
- PMID: 36574745
- PMCID: PMC9800195
- DOI: 10.1016/j.redox.2022.102591
Itaconate promotes a wound resolving phenotype in pro-inflammatory macrophages
Abstract
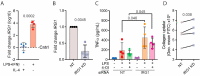
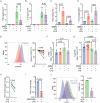
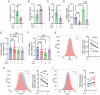
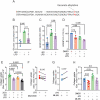
Pathological conditions associated with dysfunctional wound healing are characterized by impaired remodelling of extracellular matrix (ECM), increased macrophage infiltration, and chronic inflammation. Macrophages also play an important role in wound healing as they drive wound closure by secretion of molecules like transforming growth factor beta-1 (TGF-β). As the functions of macrophages are regulated by their metabolism, local administration of small molecules that alter this might be a novel approach for treatment of wound-healing disorders. Itaconate is a tricarboxylic acid (TCA) cycle-derived metabolite that has been associated with resolution of macrophage-mediated inflammation. However, its effects on macrophage wound healing functions are unknown. In this study, we investigated the effects of the membrane-permeable 4-octyl itaconate (4-OI) derivative on ECM scavenging by cultured human blood monocyte-derived macrophages (hMDM). We found that 4-OI reduced signalling of p38 mitogen-activated protein kinase (MAPK) induced by the canonical immune stimulus lipopolysaccharide (LPS). Likely as a consequence of this, the production of the inflammatory mediators like tumor necrosis factor (TNF)-α and cyclooxygenase (COX)-2 were also reduced. On the transcriptional level, 4-OI increased expression of the gene coding for TGF-β (TGFB1), whereas expression of the collagenase matrix metalloprotease-8 (MMP8) was reduced. Furthermore, surface levels of the anti-inflammatory marker CD36, but not CD206 and CD11c, were increased in these cells. To directly investigate the effect of 4-OI on scavenging of ECM by macrophages, we developed an assay to measure uptake of fibrous collagen. We observed that LPS promoted collagen uptake and that this was reversed by 4-OI-induced signaling of nuclear factor erythroid 2-related factor 2 (NRF2), a regulator of cellular resistance to oxidative stress and the reduced glycolytic capacity of the macrophage. These results indicate that 4-OI lowers macrophage inflammation, likely promoting a more wound-resolving phenotype.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors declare that they have no conflicts of interest.
Figures


References
-
- J V. den B., LA O., D M. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

