Kindlin2 enables EphB/ephrinB bi-directional signaling to support vascular development
- PMID: 36574991
- PMCID: PMC9795039
- DOI: 10.26508/lsa.202201800
Kindlin2 enables EphB/ephrinB bi-directional signaling to support vascular development
Abstract
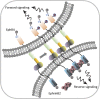
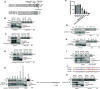
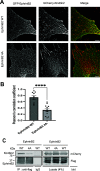
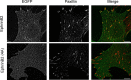
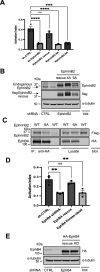
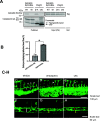
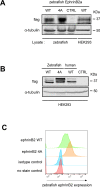
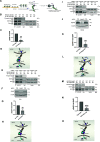
Direct contact between cells expressing either ephrin ligands or Eph receptor tyrosine kinase produces diverse developmental responses. Transmembrane ephrinB ligands play active roles in transducing bi-directional signals downstream of EphB/ephrinB interaction. However, it has not been well understood how ephrinB relays transcellular signals to neighboring cells and what intracellular effectors are involved. Here, we report that kindlin2 can mediate bi-directional ephrinB signaling through binding to a highly conserved NIYY motif in the ephrinB2 cytoplasmic tail. We show this interaction is important for EphB/ephrinB-mediated integrin activation in mammalian cells and for blood vessel morphogenesis during zebrafish development. A mixed two-cell population study revealed that kindlin2 (in ephrinB2-expressing cells) modulates transcellular EphB4 activation by promoting ephrinB2 clustering. This mechanism is also operative for EphB2/ephrinB1, suggesting that kindlin2-mediated regulation is conserved for EphB/ephrinB signaling pathways. Together, these findings show that kindlin2 enables EphB4/ephrinB2 bi-directional signal transmission.
© 2022 Li et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R (1999) Roles of ephrinB ligands and EphB receptors in cardiovascular development: Demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13: 295–306. 10.1101/gad.13.3.295 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
