Cellular senescence in cancer: clinical detection and prognostic implications
- PMID: 36575462
- PMCID: PMC9793681
- DOI: 10.1186/s13046-022-02555-3
Cellular senescence in cancer: clinical detection and prognostic implications
Abstract
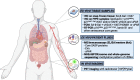
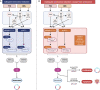
Cellular senescence is a state of stable cell-cycle arrest with secretory features in response to cellular stress. Historically, it has been considered as an endogenous evolutionary homeostatic mechanism to eliminate damaged cells, including damaged cells which are at risk of malignant transformation, thereby protecting against cancer. However, accumulation of senescent cells can cause long-term detrimental effects, mainly through the senescence-associated secretory phenotype, and paradoxically contribute to age-related diseases including cancer. Besides its role as tumor suppressor, cellular senescence is increasingly being recognized as an in vivo response in cancer patients to various anticancer therapies. Its role in cancer is ambiguous and even controversial, and senescence has recently been promoted as an emerging hallmark of cancer because of its hallmark-promoting capabilities. In addition, the prognostic implications of cellular senescence have been underappreciated due to the challenging detection and sparse in and ex vivo evidence of cellular senescence in cancer patients, which is only now catching up. In this review, we highlight the approaches and current challenges of in and ex vivo detection of cellular senescence in cancer patients, and we discuss the prognostic implications of cellular senescence based on in and ex vivo evidence in cancer patients.
Keywords: Cancer; Detection; Oncogene-induced senescence; Prognosis; SASP; Senescence; Therapy-induced senescence.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
The Senescence-Associated Secretory Phenotype: Critical Effector in Skin Cancer and Aging.J Invest Dermatol. 2016 Nov;136(11):2133-2139. doi: 10.1016/j.jid.2016.06.621. Epub 2016 Aug 17. J Invest Dermatol. 2016. PMID: 27543988 Free PMC article. Review.
-
Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases.Cancer Sci. 2017 Apr;108(4):563-569. doi: 10.1111/cas.13184. Epub 2017 Apr 18. Cancer Sci. 2017. PMID: 28165648 Free PMC article. Review.
-
Cellular Senescence: The Sought or the Unwanted?Trends Mol Med. 2018 Oct;24(10):871-885. doi: 10.1016/j.molmed.2018.08.002. Epub 2018 Aug 25. Trends Mol Med. 2018. PMID: 30153969 Review.
-
Cellular Senescence: What, Why, and How.Wounds. 2017 Jun;29(6):168-174. Wounds. 2017. PMID: 28682291 Review.
-
Quantifying Senescence-Associated Phenotypes in Primary Multipotent Mesenchymal Stromal Cell Cultures.Methods Mol Biol. 2019;2045:93-105. doi: 10.1007/7651_2019_217. Methods Mol Biol. 2019. PMID: 31020633
Cited by
-
The Role and Regulation of the NKG2D/NKG2D Ligand System in Cancer.Biology (Basel). 2023 Aug 2;12(8):1079. doi: 10.3390/biology12081079. Biology (Basel). 2023. PMID: 37626965 Free PMC article. Review.
-
Therapy-Induced Cellular Senescence: Potentiating Tumor Elimination or Driving Cancer Resistance and Recurrence?Cells. 2024 Jul 30;13(15):1281. doi: 10.3390/cells13151281. Cells. 2024. PMID: 39120312 Free PMC article. Review.
-
Investigation of differentially expressed genes related to cellular senescence between high-risk and non-high-risk groups in neuroblastoma.Front Cell Dev Biol. 2024 Jul 29;12:1421673. doi: 10.3389/fcell.2024.1421673. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39135779 Free PMC article.
-
Hedgehog pathway and cancer: A new area (Review).Oncol Rep. 2024 Sep;52(3):116. doi: 10.3892/or.2024.8775. Epub 2024 Jul 12. Oncol Rep. 2024. PMID: 38994763 Free PMC article. Review.
-
Cellular Aging and Senescence in Cancer: A Holistic Review of Cellular Fate Determinants.Aging Dis. 2024 May 21;16(3):1483-1512. doi: 10.14336/AD.2024.0421. Aging Dis. 2024. PMID: 38913050 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

