Comparison between two methods of the immediate post-placental insertion of copper intrauterine device in vaginal birth-a protocol for a randomized clinical trial
- PMID: 36575504
- PMCID: PMC9793389
- DOI: 10.1186/s13063-022-07041-x
Comparison between two methods of the immediate post-placental insertion of copper intrauterine device in vaginal birth-a protocol for a randomized clinical trial
Abstract
Background: Ensuring effective and long-term contraception in the immediate postpartum period is an effective strategy for reducing unplanned pregnancies. In the meantime, the intrauterine device (IUD) is an excellent option. The aim of our study was to evaluate the best way to insert post-placental IUDs in the immediate postpartum period. Discomfort during insertion, expulsion rate, uterine perforation rate, and proper positioning 40-60 days postpartum will be analyzed.
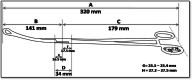
Methods: Randomized, controlled, open clinical trial. The study group will be composed of women between 18 and 43 years old who are admitted for vaginal birth at the Women's Hospital of the State University of Campinas and who wish to use the IUD as a contraceptive method. The sample will be randomized into two insertion groups: manual and forceps. To calculate the sample size, the method of comparing the proportion between 2 groups was used, setting the level of significance alpha at 5% (alpha=0.05) and the power of the sample at 80% (beta=0.20). Based on the results, it was estimated that a sample of n=186 women (n=93 with manual insertion and n=93 with forceps) would be representative for comparison of expulsion between the 2 groups. All participants will undergo a postpartum consultation 40-60 days after birth with transvaginal ultrasound to assess the proper placement of the IUD.
Discussion: Insertion of an IUD in the immediate postpartum period has been considered a good option to increase coverage and access to contraception, and its benefit outweighs the inconvenience of a higher expulsion rate.
Trial registration: This study was approved by the Ethics and Research Commission of UNICAMP (CAAE: 50497321.4.0000.5404) and the Brazilian Registry of Clinical Trials (REBEC) (number RBR-4j62jv6). This is the first version of the study protocol approved on 11/12/2021 prior to the start of participant recruitment.
Keywords: Contraception; Intrauterine device (IUD); Postpartum period; Vaginal birth, Immediate post-placental insertion.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Postpartum IUDS: keys for success.Contraception. 1992 Apr;45(4):351-61. doi: 10.1016/0010-7824(92)90057-z. Contraception. 1992. PMID: 1516367
-
Post-placental intrauterine device insertion vs puerperal insertion in women undergoing caesarean delivery in Egypt: a 1 year randomised controlled trial.Eur J Contracept Reprod Health Care. 2020 Dec;25(6):439-444. doi: 10.1080/13625187.2020.1823366. Epub 2020 Oct 2. Eur J Contracept Reprod Health Care. 2020. Retraction in: Eur J Contracept Reprod Health Care. 2023 Aug;28(4):249. doi: 10.1080/13625187.2023.2198849. PMID: 33006501 Retracted. Clinical Trial.
-
Current status of frameless anchored IUD for immediate intracesarean insertion.Dev Period Med. 2016 Jan-Mar;20(1):7-15. Dev Period Med. 2016. PMID: 27416620
-
Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis.Am J Obstet Gynecol. 2020 Aug;223(2):177-188. doi: 10.1016/j.ajog.2020.02.045. Epub 2020 Mar 3. Am J Obstet Gynecol. 2020. PMID: 32142826 Free PMC article.
-
Progestin intrauterine devices versus copper intrauterine devices for emergency contraception.Cochrane Database Syst Rev. 2023 Feb 27;2(2):CD013744. doi: 10.1002/14651858.CD013744.pub2. Cochrane Database Syst Rev. 2023. PMID: 36847591 Free PMC article.
Cited by
-
The economic impact of Long-Acting Contraceptives (LARCs) on public health.Clinics (Sao Paulo). 2025 Feb 19;80:100598. doi: 10.1016/j.clinsp.2025.100598. eCollection 2025. Clinics (Sao Paulo). 2025. PMID: 39978215 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources