Enhanced delivery of a low dose of aducanumab via FUS in 5×FAD mice, an AD model
- PMID: 36575534
- PMCID: PMC9793531
- DOI: 10.1186/s40035-022-00333-x
Enhanced delivery of a low dose of aducanumab via FUS in 5×FAD mice, an AD model
Abstract
Background: Aducanumab (Adu), which is a human IgG1 monoclonal antibody that targets oligomer and fibril forms of beta-amyloid, has been reported to reduce amyloid pathology and improve impaired cognition after administration of a high dose (10 mg/kg) of the drug in Alzheimer's disease (AD) clinical trials. The purpose of this study was to investigate the effects of a lower dose of Adu (3 mg/kg) with enhanced delivery via focused ultrasound (FUS) in an AD mouse model.
Methods: The FUS with microbubbles opened the blood-brain barrier (BBB) of the hippocampus for the delivery of Adu. The combined therapy of FUS and Adu was performed three times in total and each treatment was performed biweekly. Y-maze test, Brdu labeling, and immunohistochemical experimental methods were employed in this study. In addition, RNA sequencing and ingenuity pathway analysis were employed to investigate gene expression profiles in the hippocampi of experimental animals.
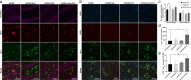
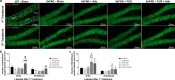
Results: The FUS-mediated BBB opening markedly increased the delivery of Adu into the brain by approximately 8.1 times in the brains. The combined treatment induced significantly less cognitive decline and decreased the level of amyloid plaques in the hippocampi of the 5×FAD mice compared with Adu or FUS alone. Combined treatment with FUS and Adu activated phagocytic microglia and increased the number of astrocytes associated with amyloid plaques in the hippocampi of 5×FAD mice. Furthermore, RNA sequencing identified that 4 enriched canonical pathways including phagosome formation, neuroinflammation signaling, CREB signaling and reelin signaling were altered in the hippocami of 5×FAD mice receiving the combined treatment.
Conclusion: In conclusion, the enhanced delivery of a low dose of Adu (3 mg/kg) via FUS decreases amyloid deposits and attenuates cognitive function deficits. FUS-mediated BBB opening increases adult hippocampal neurogenesis as well as drug delivery. We present an AD treatment strategy through the synergistic effect of the combined therapy of FUS and Adu.
Keywords: Aducanumab; Alzheimer’s disease; Focused ultrasound; Transcriptome profiling.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Dunn B, Stein P, Cavazzoni P. Approval of aducanumab for Alzheimer disease—the FDA’s perspective. JAMA Intern Med. 2021;181(10):1276–1278. - PubMed
-
- Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

