Performance and correlation of ten commercial immunoassays for the detection of SARS-CoV-2 antibodies
- PMID: 36575657
- PMCID: PMC9783098
- DOI: 10.1016/j.heliyon.2022.e12614
Performance and correlation of ten commercial immunoassays for the detection of SARS-CoV-2 antibodies
Abstract
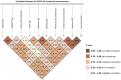
Accurate immunoassays with a good correlation to neutralizing antibodies are required to support SARS-CoV-2 diagnosis, management, vaccine deployment, and epidemiological investigation. We conducted a study to evaluate the performance and correlation of the surrogate virus neutralization test (sVNT) and other commercial immunoassays. We tested 107 sera of COVID-19 confirmed cases from three different time points, 58 confirmed non-COVID-19 sera, and 52 sera collected before the pandemic with two sVNTs, seven chemiluminescent assays, and one fluorescein assay. All assays achieved excellent sensitivity (95%-100%, ≥15 days after onset of illness), specificity (95.5%-100%), and showed moderate to high correlation with GenScript sVNT (r = 0.58 to r = 0.98), except Roche total antibodies (r = 0.48). Vazyme sVNT and Siemens total antibodies showed the highest correlation with GenScript sVNT (r = 0.98 and 0.88, respectively). Median indexes that may be used to estimate sera with the highest ability to inhibit SARS-CoV-2 and ACE-2 receptor attachment (GenScript sVNT inhibition 90%-100%) were 6.9 S/C (Abbott IgG), 161.9 COI (FREND™ IgG), 16.8 AU/ml (Snibe IgG), 40.1 S/CO (Beckman IgG), 281.9 U/ml (Mindray IgG), 712.2 U/ml (Mindray total antibodies), >10 index (Siemens total antibodies), and 95.3% inhibition (Vazyme sVNT). All ten commercial COVID-19 serology assays, with different targeting antigens, demonstrated a reliable performance, supporting the utility of those assays in clinical and research settings. However, further studies using more samples are needed to refine the results of evaluating the performances of these marketed serological assays. Reliable serological assays would be useful for clinicians, researchers and epidemiologists in confirming SARS-CoV-2 infections, observing SARS-CoV-2 transmission, and immune response post infection and vaccination, leading to better management and control of the disease.
Keywords: Antibodies; Immunoassays; SARS-CoV-2; sVNT.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bennett R.S., Postnikova E.N., Liang J., Gross R., Mazur S., Dixit S., Lukin V.V., Kocher G., Yu S., Georgia-Clark S., Gerhardt D., Cai Y., Marron L., Holbrook M.R. bioRxiv; 2021. 'Scalable, Micro-neutralization Assay for Qualitative Assessment of SARS-CoV-2 (COVID-19) Virus-Neutralizing Antibodies in Human Clinical Samples. - PMC - PubMed
-
- Burbelo P.D., Riedo F.X., Morishima C., Rawlings S., Smith D., Das S., Strich J.R., Chertow D.S., Davey R.T., Cohen J.I. 'Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J. Infect. Dis. 2020;222:206–213. - PMC - PubMed
-
- Dorschug A., Frickmann H., Schwanbeck J., Yilmaz E., Mese K., Hahn A., Gross U., Zautner A.E. Diagnostics; Basel): 2021. 'Comparative Assessment of Sera from Individuals after S-Gene RNA-Based SARS-CoV-2 Vaccination with Spike-Protein-Based and Nucleocapsid-Based Serological Assays; p. 11. - PMC - PubMed
-
- Edouard S., Colson P., Melenotte C., Di Pinto F., Thomas L., La Scola B., Million M., Tissot-Dupont H., Gautret P., Stein A., Brouqui P., Parola P., Lagier J.C., Raoult D., Drancourt M. Evaluating the serological status of COVID-19 patients using an indirect immunofluorescent assay, France. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:361–371. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

