Targeted Therapy for Locally Advanced or Metastatic Urothelial Cancer (mUC): Therapeutic Potential of Sacituzumab Govitecan
- PMID: 36575731
- PMCID: PMC9790156
- DOI: 10.2147/OTT.S339348
Targeted Therapy for Locally Advanced or Metastatic Urothelial Cancer (mUC): Therapeutic Potential of Sacituzumab Govitecan
Abstract
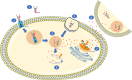
Urothelial carcinoma is the second most frequent genitourinary malignancy. Despite the poor prognosis, new treatment options have emerged and have expanded the therapeutic landscape for the disease. Although major improvements have been achieved, many patients experience rapid disease progression and low responses in subsequent lines of therapy. Sacituzumab govitecan is an ADC that targets Trop-2, which is highly expressed in urothelial cancers. Promising results in early clinical trials have led to further drug development which confirmed encouraging efficacy. Sacituzumab govitecan has been given accelerated approval in 2021 for patients with locally advanced and metastatic urothelial cancer who previously received a platinum containing chemotherapy and either a programmed death receptor-1 or programmed death ligand inhibitor. The results are promising, with encouraging efficacy and safety, however responses are not universal. There is a growing comprehension of mechanisms of resistance and predictive biomarkers that are crucial to improving outcomes. In this review, we summarize the current knowledge on antibody-drug conjugates and the clinical findings that led to the approval of Sacituzumab govitecan and discuss the therapeutic potential of new combinations, mechanisms of resistance and predictive biomarkers.
Keywords: ADC; SN-38; Trop-2; antibody–drug conjugate; bladder cancer; human trophoblast cell surface antigen 2.
© 2022 Fontes et al.
Conflict of interest statement
Dr Mariane S Fontes reports personal fees and travel expenses from Janssen Brazil, Astellas, MSD, AstraZeneca, Zodiac; personal fees from Bayer and Amgen, during the conduct of the study. Dr Daniel Vargas Pivato de Almeida reports personal fees from Astellas, AstraZeneca, Bayer, Eli Lilly, Ipsen, Janssen-Cilag, and Sanofi-Aventis, outside the submitted work. Dr Clarissa Cavalin reports personal fees and/or non-financial support from Bayer, Eli Lilly, AstraZeneca, and Novartis, outside the submitted work. Dr Scott T Tagawa reports grants and/or personal fees from Gilead, Seagen, Janssen, EMD Serono, and Merck, outside the submitted work. In addition, Dr Scott T Tagawa has a patent (biomarkers for sacituzumab govitecan therapy [Immunomedics / Gilead / Weill Cornell) issued to Gilead. The authors report no other conflicts of interest in this work.
Figures
References
-
- Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39(22):2474–2485. doi:10.1200/JCO.20.03489 - DOI - PMC - PubMed
-
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi:10.1016/S1470-2045(17)30616-2 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous