Safety and immunogenicity of a combined DTacP-sIPV-Hib vaccine in animal models
- PMID: 36576263
- PMCID: PMC9891680
- DOI: 10.1080/21645515.2022.2160158
Safety and immunogenicity of a combined DTacP-sIPV-Hib vaccine in animal models
Abstract
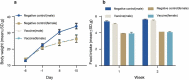
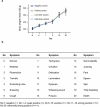
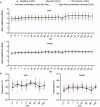
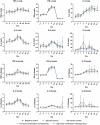
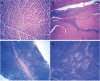
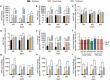
The DTacP-sIPV-Hib combination vaccine can replace the single-component acellular pertussis, diphtheria, tetanus, polio, and Haemophilus influenzae type B vaccines. In this study, we evaluated the safety and immunogenicity of a newly developed DTacP-sIPV-Hib combination vaccine in animal models. We used 40 mice and 46 cynomolgus monkeys to evaluate acute and long-term toxicity. Thirty-six guinea pigs were used for sensitization assessment. For immunogenicity assessment, 50 NIH mice and 50 rats were equally randomized to receive 3 doses of 3 different batches of the tested vaccine at an interval of 21 d, or physiological saline solution (0.5 mL). Orbital blood was collected at an interval of 21 d post inoculation to detect related antibody titers or neutralizing antibody titers against poliovirus. Gross autopsy and histopathological examination revealed no abnormal toxicity or irritation in mice and cynomolgus monkeys. Sensitization assessment in guinea pigs indicated the lack of evident allergic symptoms in the high- and low-dose vaccine groups within 30 min after repeated stimulation. The DTacP-sIPV-Hib combination vaccine induced significant immune responses in mice, rats, and cynomolgus monkeys, with 100% seroconversion rates after 3 doses. The DTacP-sIPV-Hib combination vaccine is safe and immunogenic in animal models. Three doses of the vaccine elicited satisfactory antibody responses in mice, rats, and cynomolgus monkeys.
Keywords: DTacP-sIPV-Hib vaccine; Safety; animal model; immunogenicity.
Conflict of interest statement
All the authors are employees of the manufacturer of the DTacP-sIPV-Hib combination vaccine subject of the manuscript.
Figures

References
-
- Bar-On ES, Goldberg E, Hellmann S, Leibovici L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database Syst Rev. 2012;4:CD005530. doi: 10.1002/14651858.CD005530.pub3. - DOI - PMC - PubMed
-
- Stanley A P, Walter A O, Paul A O, Kathryn M E. Section 2: Licensed Vaccines and Vaccines in Develpment. 15. Combination vaccines. Plotkin’s vaccines. 7th ed. Philadelphia, PA: Elsevier; 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
