In vitro and ex vivo modeling of enteric bacterial infections
- PMID: 36576310
- PMCID: PMC9809952
- DOI: 10.1080/19490976.2022.2158034
In vitro and ex vivo modeling of enteric bacterial infections
Abstract
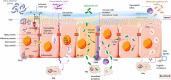
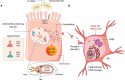
Enteric bacterial infections contribute substantially to global disease burden and mortality, particularly in the developing world. In vitro 2D monolayer cultures have provided critical insights into the fundamental virulence mechanisms of a multitude of pathogens, including Salmonella enterica serovars Typhimurium and Typhi, Vibrio cholerae, Shigella spp., Escherichia coli and Campylobacter jejuni, which have led to the identification of novel targets for antimicrobial therapy and vaccines. In recent years, the arsenal of experimental systems to study intestinal infections has been expanded by a multitude of more complex models, which have allowed to evaluate the effects of additional physiological and biological parameters on infectivity. Organoids recapitulate the cellular complexity of the human intestinal epithelium while 3D bioengineered scaffolds and microphysiological devices allow to emulate oxygen gradients, flow and peristalsis, as well as the formation and maintenance of stable and physiologically relevant microbial diversity. Additionally, advancements in ex vivo cultures and intravital imaging have opened new possibilities to study the effects of enteric pathogens on fluid secretion, barrier integrity and immune cell surveillance in the intact intestine. This review aims to present a balanced and updated overview of current intestinal in vitro and ex vivo methods for modeling of enteric bacterial infections. We conclude that the different paradigms are complements rather than replacements and their combined use promises to further our understanding of host-microbe interactions and their impacts on intestinal health.
Keywords: Organotypic culture; infection models; intestine; microphysiological systems; monolayer cell culture.
Conflict of interest statement
VML is CEO and shareholder of HepaPredict AB, as well as co-founder and shareholder of PersoMedix AB. The other authors declare no conflicts of interest.
Figures


Similar articles
-
A Versatile Human Intestinal Organoid-Derived Epithelial Monolayer Model for the Study of Enteric Pathogens.Microbiol Spectr. 2021 Sep 3;9(1):e0000321. doi: 10.1128/Spectrum.00003-21. Epub 2021 Jun 9. Microbiol Spectr. 2021. PMID: 34106568 Free PMC article.
-
The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection.Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0008-2014. Microbiol Spectr. 2015. PMID: 26185088
-
Salmonella enterica Serovar Typhimurium SPI-1 and SPI-2 Shape the Global Transcriptional Landscape in a Human Intestinal Organoid Model System.mBio. 2021 May 18;12(3):e00399-21. doi: 10.1128/mBio.00399-21. mBio. 2021. PMID: 34006652 Free PMC article.
-
The Impact of Oxygen on Bacterial Enteric Pathogens.Adv Appl Microbiol. 2016;95:179-204. doi: 10.1016/bs.aambs.2016.04.002. Epub 2016 May 18. Adv Appl Microbiol. 2016. PMID: 27261784 Review.
-
Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens.Toxins (Basel). 2017 Feb 10;9(2):60. doi: 10.3390/toxins9020060. Toxins (Basel). 2017. PMID: 28208612 Free PMC article. Review.
Cited by
-
An Open-Source 3D-Printed Recording Stage with Customizable Chambers for Ex Vivo Experiments.eNeuro. 2024 Sep 13;11(9):ENEURO.0257-24.2024. doi: 10.1523/ENEURO.0257-24.2024. Print 2024 Sep. eNeuro. 2024. PMID: 39197950 Free PMC article.
-
Current methodologies available to evaluate the virulence potential among Listeria monocytogenes clonal complexes.Front Microbiol. 2024 Oct 10;15:1425437. doi: 10.3389/fmicb.2024.1425437. eCollection 2024. Front Microbiol. 2024. PMID: 39493856 Free PMC article. Review.
-
Advancements in understanding bacterial enteritis pathogenesis through organoids.Mol Biol Rep. 2024 Apr 15;51(1):512. doi: 10.1007/s11033-024-09495-5. Mol Biol Rep. 2024. PMID: 38622483 Review.
-
Study Models for Chlamydia trachomatis Infection of the Female Reproductive Tract.Microorganisms. 2025 Feb 28;13(3):553. doi: 10.3390/microorganisms13030553. Microorganisms. 2025. PMID: 40142446 Free PMC article. Review.
-
Experimental Approaches to Visualize Effector Protein Translocation During Host-Pathogen Interactions.Bioessays. 2025 Apr;47(4):e202400188. doi: 10.1002/bies.202400188. Epub 2025 Mar 13. Bioessays. 2025. PMID: 40078034 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
