Zebrafish model of RERE syndrome recapitulates key ophthalmic defects that are rescued by small molecule inhibitor of shh signaling
- PMID: 36576487
- PMCID: PMC11528340
- DOI: 10.1002/dvdy.561
Zebrafish model of RERE syndrome recapitulates key ophthalmic defects that are rescued by small molecule inhibitor of shh signaling
Abstract
Background: RERE is a highly conserved transcriptional co-regulator that is associated with a human neurodevelopmental disorder with or without anomalies of the brain, eye, or heart (NEDBEH, OMIM: 616975).
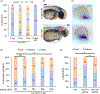
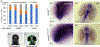
Results: We show that the zebrafish rerea mutant (babyface) robustly recapitulates optic fissure closure defects resulting from loss of RERE function, as observed in humans. These defects result from expansion of proximal retinal optic stalk (OS) and reduced expression of some of the ventral retinal fate genes due to deregulated protein signaling. Using zebrafish and cell-based assays, we determined that NEDBEH-associated human RERE variants function as hypomorphs in their ability to repress shh signaling and some exhibit abnormal nuclear localization. Inhibiting shh signaling by the protein inhibitor HPI-1 rescues coloboma, confirming our observation that coloboma in rerea mutants is indeed due to deregulation of shh signaling.
Conclusions: Zebrafish rerea mutants exhibit OS and optic fissure closure defects. The optic fissure closure defect was rescued by an shh signaling inhibitor, suggesting that this defect could arise due to deregulated shh signaling.
Keywords: RERE and SHH; coloboma; optic fissure; optic stalk.
Published 2022. This article is a U.S. Government work and is in the public domain in the USA. Developmental Dynamics published by Wiley Periodicals LLC on behalf of American Association for Anatomy.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Figures





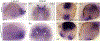
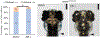
Similar articles
-
Increased Netrin downstream of overactive Hedgehog signaling disrupts optic fissure formation.Dev Dyn. 2025 Feb;254(2):158-173. doi: 10.1002/dvdy.733. Epub 2024 Aug 21. Dev Dyn. 2025. PMID: 39166841 Free PMC article.
-
Sox4 regulates choroid fissure closure by limiting Hedgehog signaling during ocular morphogenesis.Dev Biol. 2015 Mar 1;399(1):139-153. doi: 10.1016/j.ydbio.2014.12.026. Epub 2014 Dec 31. Dev Biol. 2015. PMID: 25557621 Free PMC article.
-
Zebrafish blowout provides genetic evidence for Patched1-mediated negative regulation of Hedgehog signaling within the proximal optic vesicle of the vertebrate eye.Dev Biol. 2008 Jul 1;319(1):10-22. doi: 10.1016/j.ydbio.2008.03.035. Epub 2008 Apr 4. Dev Biol. 2008. PMID: 18479681 Free PMC article.
-
Aberrant forebrain signaling during early development underlies the generation of holoprosencephaly and coloboma.Biochim Biophys Acta. 2011 Mar;1812(3):390-401. doi: 10.1016/j.bbadis.2010.09.005. Epub 2010 Sep 16. Biochim Biophys Acta. 2011. PMID: 20850526 Review.
-
Genes and pathways in optic fissure closure.Semin Cell Dev Biol. 2019 Jul;91:55-65. doi: 10.1016/j.semcdb.2017.10.010. Epub 2017 Dec 6. Semin Cell Dev Biol. 2019. PMID: 29198497 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

