Molecular response patterns in relapsed/refractory AML patients treated with selinexor and chemotherapy
- PMID: 36576532
- PMCID: PMC9889470
- DOI: 10.1007/s00277-022-05075-4
Molecular response patterns in relapsed/refractory AML patients treated with selinexor and chemotherapy
Abstract
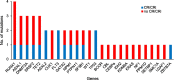
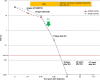
Relapse in patients with acute myeloid leukemia (AML) is common and is associated with a dismal prognosis. Treatment options are limited and the understanding of molecular response patterns is still challenging. We analyzed the clonal response patterns of 15 patients with relapsed/refractory AML treated with selinexor in a phase II trial (SAIL). DNA was analyzed at three time points and showed a decline of mutated alleles in FLT3, SF3B1, and TP53 under SAIL treatment. Overall survival (OS) was similar between patients with declining versus persisting clones. We show an interesting long-term course of a patient who relapsed after allogeneic stem cell transplantation (alloHCT) with SF3B1- and SRSF2-mutated AML and received selinexor as maintenance treatment for 4 years. Measurable residual disease (MRD) remained detectable for 2 weeks after donor lymphocyte infusion (DLI) in this patient and then remained negative under selinexor maintenance treatment. Selinexor was tolerated well and was stopped after 4 years of SAIL treatment. We present an exploratory study and identify subclonal patterns of patients treated with selinexor.
Keywords: AML; Molecular patterns; Relapse; Selinexor.
© 2022. The Author(s).
Conflict of interest statement
The institutions of WF and MH received research funding from Karyopharm. The other authors have no potential conflicts of interest.
Figures
References
-
- Ranganathan P, Kashyap T, Yu X, Meng X, Lai TH, McNeil B, et al. XPO1 inhibition using selinexor synergizes with chemotherapy in acute myeloid leukemia by targeting DNA repair and restoring topoisomerase IIα to the nucleus. Clin Cancer Res. 2016;22(24):6142–6152. doi: 10.1158/1078-0432.CCR-15-2885. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

