Predictive Utility of a Coronary Artery Disease Polygenic Risk Score in Primary Prevention
- PMID: 36576811
- PMCID: PMC9857431
- DOI: 10.1001/jamacardio.2022.4466
Predictive Utility of a Coronary Artery Disease Polygenic Risk Score in Primary Prevention
Abstract
Importance: The clinical utility of polygenic risk scores (PRS) for coronary artery disease (CAD) has not yet been established.
Objective: To investigate the ability of a CAD PRS to potentially guide statin initiation in primary prevention after accounting for age and clinical risk.
Design, setting, and participants: This was a longitudinal cohort study with enrollment starting on January 1, 2006, and ending on December 31, 2010, with data updated to mid-2021, using data from the UK Biobank, a long-term population study of UK citizens. A replication analysis was performed in Biobank Japan. The analysis included all patients without a history of CAD and who were not taking lipid-lowering therapy. Data were analyzed from January 1 to June 30, 2022.
Exposures: Polygenic risk for CAD was defined as low (bottom 20%), intermediate, and high (top 20%) using a CAD PRS including 241 genome-wide significant single-nucleotide variations (SNVs). The pooled cohort equations were used to estimate 10-year atherosclerotic cardiovascular disease (ASCVD) risk and classify individuals as low (<5%), borderline (5-<7.5%), intermediate (7.5-<20%), or high risk (≥20%).
Main outcomes and measures: Myocardial infarction (MI) and ASCVD events (defined as incident clinical CAD [including MI], stroke, or CV death).
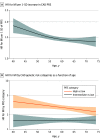
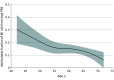
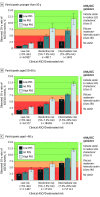
Results: A total of 330 201 patients (median [IQR] age, 57 [40-74] years; 189 107 female individuals [57%]) were included from the UK Biobank. Over the 10-year follow-up, 4454 individuals had an MI. The CAD PRS was significantly associated with the risk of MI in all age groups but had significantly stronger risk prediction at younger ages (age <50 years: hazard ratio [HR] per 1 SD of PRS, 1.72; 95% CI, 1.56-1.89; age 50-60 years: HR, 1.46; 95% CI, 1.38-1.53; age >60 years: HR, 1.42; 95% CI, 1.37-1.48; P for interaction <.001). In patients younger than 50 years, those with high PRS had a 3- to 4-fold increased associated risk of MI compared with those in the low PRS category. A significant interaction between CAD PRS and age was replicated in Biobank Japan. When CAD PRS testing was added to the clinical ASCVD risk score in individuals younger than 50 years, 591 of 4373 patients (20%) with borderline risk were risk stratified into intermediate risk, warranting initiation of statin therapy and 3198 of 7477 patients (20%) with both borderline or intermediate risk were stratified as low risk, thus not warranting therapy.
Conclusions and relevance: Results of this cohort study suggest that the predictive ability of a CAD PRS was greater in younger individuals and can be used to better identify patients with borderline and intermediate clinical risk who should initiate statin therapy.
Conflict of interest statement
Figures
Comment in
-
Cardiovascular Disease Risk Prediction in Young Adults-The Next Frontier.JAMA Cardiol. 2023 Feb 1;8(2):137-138. doi: 10.1001/jamacardio.2022.4887. JAMA Cardiol. 2023. PMID: 36576809 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

