Modular Synthesis of Unnatural Peptides via Rh(III)-Catalyzed Diastereoselective Three-Component Carboamidation Reaction
- PMID: 36576945
- PMCID: PMC10580301
- DOI: 10.1021/jacs.2c10793
Modular Synthesis of Unnatural Peptides via Rh(III)-Catalyzed Diastereoselective Three-Component Carboamidation Reaction
Abstract
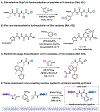
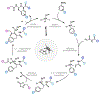
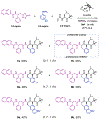
Herein we report a modular peptide ligation methodology that couples dioxazolones, arylboronic acids, and acrylamides to construct amide bonds in a diastereoselective manner under mild conditions, facilitated by Rh(III) catalysis. By converting the C-terminus of one peptide into a dioxazolone and the N-terminus of a second peptide into an acrylamide, the two pieces can be bridged by an arylboronic acid to construct unnatural phenylalanine, tyrosine, and tryptophan residues at the junction point with diastereoselectivity for their corresponding d-stereocenters. The reaction exhibits excellent functional group tolerance with a large substrate scope and is compatible with a wide array of protected amino acid residues that are utilized in Fmoc solid phase peptide synthesis. The methodology is applied to the synthesis of six diastereomeric proteasome inhibitor analogs, as well as the ligation of two 10-mer oligopeptides to construct a 21-mer polypeptide with an unnatural phenylalanine residue at the center.
Figures


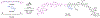
Similar articles
-
Crafting Unnatural Peptide Macrocycles via Rh(III)-Catalyzed Carboamidation.J Am Chem Soc. 2024 Jul 31;146(30):20868-20877. doi: 10.1021/jacs.4c05248. Epub 2024 Jul 18. J Am Chem Soc. 2024. PMID: 39024122
-
Convenient route to Fmoc-homotyrosine via metallaphotoredox catalysis and its use in the total synthesis of anabaenopeptin cyclic peptides.Org Biomol Chem. 2023 Nov 22;21(45):9011-9020. doi: 10.1039/d3ob01608k. Org Biomol Chem. 2023. PMID: 37921761
-
Quaternary β2,2 -Amino Acids: Catalytic Asymmetric Synthesis and Incorporation into Peptides by Fmoc-Based Solid-Phase Peptide Synthesis.Angew Chem Int Ed Engl. 2018 Jan 15;57(3):818-822. doi: 10.1002/anie.201711143. Epub 2017 Dec 14. Angew Chem Int Ed Engl. 2018. PMID: 29168280
-
[Asymmetric Synthesis of Unnatural Amino Acid-containing Peptides via Direct Asymmetric Reaction of Peptidyl Compounds].Yakugaku Zasshi. 2018;138(11):1371-1379. doi: 10.1248/yakushi.18-00143. Yakugaku Zasshi. 2018. PMID: 30381645 Review. Japanese.
-
Some of the amino acid chemistry going on in the Laboratory of Amino Acids, Peptides and Proteins.Amino Acids. 1999;16(3-4):345-79. doi: 10.1007/BF01388176. Amino Acids. 1999. PMID: 10399020 Review.
Cited by
-
Photoinduced Copper-Catalyzed Late-Stage Azidoarylation of Alkenes via Arylthianthrenium Salts.J Am Chem Soc. 2023 Jun 28;145(25):13542-13548. doi: 10.1021/jacs.3c04016. Epub 2023 Jun 12. J Am Chem Soc. 2023. PMID: 37307146 Free PMC article.
-
Expedient and divergent synthesis of unnatural peptides through cobalt-catalyzed diastereoselective umpolung hydrogenation.Sci Adv. 2023 Dec 22;9(51):eadk4950. doi: 10.1126/sciadv.adk4950. Epub 2023 Dec 20. Sci Adv. 2023. PMID: 38117889 Free PMC article.
-
Transition Metal-Free Difunctionalization of Unactivated Alkenes: Arylation/Azidation, Arylation/Chlorination, and Arylation/Cyanation.Chem. 2024 Oct 10;10(10):3243-3253. doi: 10.1016/j.chempr.2024.07.036. Epub 2024 Sep 2. Chem. 2024. PMID: 39677497
-
Transition Metal Catalysis for the Asymmetric Synthesis of 2-Arylethylamines: A Review of the New Millennium.Molecules. 2025 Apr 11;30(8):1721. doi: 10.3390/molecules30081721. Molecules. 2025. PMID: 40333644 Free PMC article. Review.
References
-
- Li Petri G; Di Martino S; De Rosa M Peptidomimetics: An Overview of Recent Medicinal Chemistry Efforts toward the Discovery of Novel Small Molecule Inhibitors. J. Med. Chem 2022, 65 (11), 7438–7475. - PubMed
-
- Fosgerau K; Hoffmann T Peptide Therapeutics: Current Status and Future Directions. Drug Discov. 2015, 20 (1), 122–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

