VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19
- PMID: 36577095
- PMCID: PMC9812289
- DOI: 10.1056/NEJMoa2208822
VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19
Abstract
Background: Nirmatrelvir-ritonavir has been authorized for emergency use by many countries for the treatment of coronavirus disease 2019 (Covid-19). However, the supply falls short of the global demand, which creates a need for more options. VV116 is an oral antiviral agent with potent activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
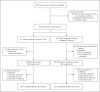
Methods: We conducted a phase 3, noninferiority, observer-blinded, randomized trial during the outbreak caused by the B.1.1.529 (omicron) variant of SARS-CoV-2. Symptomatic adults with mild-to-moderate Covid-19 with a high risk of progression were assigned to receive a 5-day course of either VV116 or nirmatrelvir-ritonavir. The primary end point was the time to sustained clinical recovery through day 28. Sustained clinical recovery was defined as the alleviation of all Covid-19-related target symptoms to a total score of 0 or 1 for the sum of each symptom (on a scale from 0 to 3, with higher scores indicating greater severity; total scores on the 11-item scale range from 0 to 33) for 2 consecutive days. A lower boundary of the two-sided 95% confidence interval for the hazard ratio of more than 0.8 was considered to indicate noninferiority (with a hazard ratio of >1 indicating a shorter time to sustained clinical recovery with VV116 than with nirmatrelvir-ritonavir).
Results: A total of 822 participants underwent randomization, and 771 received VV116 (384 participants) or nirmatrelvir-ritonavir (387 participants). The noninferiority of VV116 to nirmatrelvir-ritonavir with respect to the time to sustained clinical recovery was established in the primary analysis (hazard ratio, 1.17; 95% confidence interval [CI], 1.01 to 1.35) and was maintained in the final analysis (median, 4 days with VV116 and 5 days with nirmatrelvir-ritonavir; hazard ratio, 1.17; 95% CI, 1.02 to 1.36). In the final analysis, the time to sustained symptom resolution (score of 0 for each of the 11 Covid-19-related target symptoms for 2 consecutive days) and to a first negative SARS-CoV-2 test did not differ substantially between the two groups. No participants in either group had died or had had progression to severe Covid-19 by day 28. The incidence of adverse events was lower in the VV116 group than in the nirmatrelvir-ritonavir group (67.4% vs. 77.3%).
Conclusions: Among adults with mild-to-moderate Covid-19 who were at risk for progression, VV116 was noninferior to nirmatrelvir-ritonavir with respect to the time to sustained clinical recovery, with fewer safety concerns. (Funded by Vigonvita Life Sciences and others; ClinicalTrials.gov number, NCT05341609; Chinese Clinical Trial Registry number, ChiCTR2200057856.).
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
VV116 or Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19.N Engl J Med. 2023 Jun 22;388(25):2395. doi: 10.1056/NEJMc2305030. N Engl J Med. 2023. PMID: 37342930 No abstract available.
-
VV116 or Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19.N Engl J Med. 2023 Jun 22;388(25):2395. doi: 10.1056/NEJMc2305030. N Engl J Med. 2023. PMID: 37342931 No abstract available.
-
VV116 or Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19.N Engl J Med. 2023 Jun 22;388(25):2395-2396. doi: 10.1056/NEJMc2305030. N Engl J Med. 2023. PMID: 37342932 No abstract available.
-
VV116 or Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19.N Engl J Med. 2023 Jun 22;388(25):2396. doi: 10.1056/NEJMc2305030. N Engl J Med. 2023. PMID: 37342933 No abstract available.
-
VV116 or Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19. Reply.N Engl J Med. 2023 Jun 22;388(25):2396-2397. doi: 10.1056/NEJMc2305030. N Engl J Med. 2023. PMID: 37342934 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
