Progression patterns and therapeutic sequencing following immune checkpoint inhibition for hepatocellular carcinoma: An international observational study
- PMID: 36577703
- PMCID: PMC10947007
- DOI: 10.1111/liv.15502
Progression patterns and therapeutic sequencing following immune checkpoint inhibition for hepatocellular carcinoma: An international observational study
Abstract
Background and aims: Different approaches are available after the progression of disease (PD) to immune checkpoint inhibitors (ICIs) for hepatocellular carcinoma (HCC), including the continuation of ICI, treatment switching to tyrosine kinase inhibitors (TKIs) and cessation of anticancer therapy. We sought to characterise the relationship between radiological patterns of progression and survival post-ICI, also appraising treatment strategies.
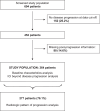
Methods: We screened 604 HCC patients treated with ICIs, including only those who experienced PD by data cut-off. We evaluated post-progression survival (PPS) according to the treatment strategy at PD and verified its relationship with radiological patterns of progression: intrahepatic growth (IHG), new intrahepatic lesion (NIH), extrahepatic growth (EHG), new extrahepatic lesion (NEH) and new vascular invasion (nVI).
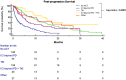
Results: Of 604 patients, 364 (60.3%) experienced PD during observation. Median PPS was 5.3 months (95% CI: 4.4-6.9; 271 events). At the data cut-off, 165 patients (45%) received no post-progression anticancer therapy; 64 patients (17.6%) continued ICI beyond PD. IHG (HR 1.64 [95% CI: 1.21-2.22]; p = .0013) and nVI (HR 2.15 [95% CI: 1.38-3.35]; p = .0007) were associated with shorter PPS. Multivariate models adjusted for progression patterns, treatment line and albumin-bilirubin grade and Eastern Cooperative Oncology Group performance status at PD confirmed receipt of ICI beyond PD with (HR 0.17, 95% CI: 0.09-0.32; p < .0001) or without subsequent TKI (HR 0.39, 95% CI: 0.26-0.58; p < .0001) as predictors of prolonged PPS versus no anticancer therapy.
Conclusions: ICI-TKI sequencing is a consolidated option in advanced HCC. nVI and IHG predict a poorer prognosis. Despite lack of recommendation, the continuation of ICI beyond progression in HCC is adopted clinically: future efforts should appraise which patients benefit from this approach.
© 2022 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
M. Pinter is an investigator for Bayer, BMS, Lilly and Roche; he received speaker honoraria from Bayer, BMS, Eisai, Lilly and MSD; he is a consultant for Bayer, BMS, Eisai, Ipsen, Lilly, MSD and Roche; he received travel support from Bayer and BMS. B. Scheiner received travel support from Gilead, Ipsen and AbbVie. T.U. Marron serves on advisory and/or data safety boards for Rockefeller University, Regeneron, BMS, Boehringer Ingelheim, Atara, AstraZeneca, Genentech, Celldex, Surface, NGMbio, DBV Technologies and receives research funding from Regeneron, Bristol‐Myers Squibb, Merck, Boehringer Ingelheim. T. Jun owns stock and is employed by Sema4. D. Bettinger has received lecture and speaker fees from Bayer Healthcare, the Falk Foundation Germany and consulting fees from Boston Scientific. T. Pressiani received consulting fees from Bayer; and institutional research funding from Bayer, Lilly and Roche. N. Personeni received consulting fees from Amgen, Merck Serono and Servier; lectures fees from AbbVie, Gilead, Lilly and Sanofi; travel expenses from Amgen, ArQule and institutional research funding from Basilea, Merck Serono and Servier. L. Rimassa received consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Servier, Taiho Oncology, Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi; travel expenses from Ipsen and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Zymeworks. A. Vogel reports honoraria for speaker, consultancy and advisory role from Roche, AstraZeneca, EISAI, Bayer, Merck, Bristol Myers Squibb, Merck Sharp & Dohme, Incyte, PierreFabre, Ipsen and Sanofi. P. R. Galle reports a consulting or advisory role and received honoraria from AdaptImmune, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Lilly, Merck Sharp & Dohme, Roche and Sirtex; has been on a speakers bureau for AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Lilly, Merck Sharp & Dohme, Roche and Sirtex; has received research funding from Bayer and Roche; has provided expert testimony for Lilly and has received travel or accommodation expenses from AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Lilly, and Roche. A. Cortellini received grant consultancies and speaker fees from AstraZeneca, BMS, Roche, MSD, Novartis, Eisai outside the submitted work. D. J. Pinato received lecture fees from ViiV Healthcare and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche and Astra Zeneca; received research funding (to institution) from MSD and BMS. All remaining authors have declared no conflict of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilised in the production of this manuscript.
Figures

Comment in
-
The prognostic role of tumour progression and liver function at progression under immunotherapy for hepatocellular carcinoma.Liver Int. 2023 Mar;43(3):528-530. doi: 10.1111/liv.15513. Liver Int. 2023. PMID: 36808694 No abstract available.
References
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378‐390. - PubMed
-
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894‐1905. - PubMed
-
- Finn RS, Qin S, Ikeda M, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open‐label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_suppl):267.
-
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;389(10064):56‐66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

