The safety of an MRI simulation-guided boost after short-course preoperative radiotherapy for unresectable rectal cancer (SUNRISE): interim analysis of a randomized phase II trial
- PMID: 36578032
- PMCID: PMC9795765
- DOI: 10.1186/s13014-022-02182-4
The safety of an MRI simulation-guided boost after short-course preoperative radiotherapy for unresectable rectal cancer (SUNRISE): interim analysis of a randomized phase II trial
Abstract
Purpose: The safety of an MRI simulation-guided boost after short-course preoperative radiotherapy (SCPRT) for unresectable rectal cancer is assessed with a planned interim analysis.
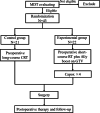
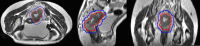
Methods and materials: Patients diagnosed with clinical stage T3-4 or regional lymph node-positive disease with positive mesorectal fascia or T4b disease evaluated by pelvic MRI were randomly assigned to the SCPRT-boost group (25 Gy in 5 fractions plus 4 Gy delivered to the gross tumor volume, followed by four cycles of chemotherapy) or preoperative chemoradiotherapy group (50 Gy in 25 fractions with concurrent chemotherapy). Then, patients received total mesorectal excision surgery after preoperative treatment. The primary endpoint was the R0 resection rate. The interim analysis was performed when 42 patients completed their assigned treatments.
Results: From October 2018 to November 2019, a total of 43 patients were enrolled, and 42 patients were included in the interim analysis. During preoperative therapy, grade 3 or above toxicities were observed in 10/21 (47.6%) patients in the experimental group, and 4/21 (19.0%) patients in the control group. A total of 17 (81.0%) and 13 (61.9%) patients in the experimental group and control group underwent surgery, respectively. Overall, 65.1% of the patients achieved R0 resection in the intention-to-treat analysis. Surgery-related adverse complications were observed in 2 patients (11.8%) in the experimental group and 1 patient (7.7%) in the control group.
Conclusion: Our results show that the toxicity of an MRI simulation-guided boost after SCPRT for unresectable rectal cancer is acceptable. Thus, this clinical trial will be continued as planned.
Keywords: MRI; Preoperative; Radiotherapy boost; Rectal cancer.
© 2022. The Author(s).
Conflict of interest statement
There are neither relationships with industry and organizations in this study, nor potential conflicts of interest between authors and other persons.
Figures
Similar articles
-
Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial.Lancet Oncol. 2021 Jan;22(1):29-42. doi: 10.1016/S1470-2045(20)30555-6. Epub 2020 Dec 7. Lancet Oncol. 2021. PMID: 33301740 Clinical Trial.
-
Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial.Lancet Oncol. 2015 Aug;16(8):979-89. doi: 10.1016/S1470-2045(15)00159-X. Epub 2015 Jul 15. Lancet Oncol. 2015. PMID: 26189067 Clinical Trial.
-
[Analysis on efficacy and safety of total neoadjuvant therapy in patients with locally advanced rectal cancer with high risk factors].Zhonghua Wei Chang Wai Ke Za Zhi. 2019 Apr 25;22(4):349-356. doi: 10.3760/cma.j.issn.1671-0274.2019.04.007. Zhonghua Wei Chang Wai Ke Za Zhi. 2019. PMID: 31054549 Chinese.
-
Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study.Int J Radiat Oncol Biol Phys. 2006 Mar 15;64(4):1129-39. doi: 10.1016/j.ijrobp.2005.09.017. Epub 2006 Jan 18. Int J Radiat Oncol Biol Phys. 2006. PMID: 16414206 Clinical Trial.
-
High dose rate brachytherapy as a boost after preoperative chemoradiotherapy for more advanced rectal tumours: the Clatterbridge experience.Clin Oncol (R Coll Radiol). 2007 Nov;19(9):711-9. doi: 10.1016/j.clon.2007.07.018. Epub 2007 Sep 19. Clin Oncol (R Coll Radiol). 2007. PMID: 17884396 Review.
Cited by
-
THeragnostic utilities for neoplastic DisEases of the rectum by MRI guided radiotherapy (THUNDER 2) phase II trial: interim safety analysis.Radiat Oncol. 2023 Oct 6;18(1):163. doi: 10.1186/s13014-023-02353-x. Radiat Oncol. 2023. PMID: 37803322 Free PMC article. Clinical Trial.
-
Efficacy and safety of MR-guided adaptive simultaneous integrated boost radiotherapy to primary lesions and positive lymph nodes in the neoadjuvant treatment of locally advanced rectal cancer: a randomized controlled phase III trial.Radiat Oncol. 2024 Sep 12;19(1):118. doi: 10.1186/s13014-024-02506-6. Radiat Oncol. 2024. PMID: 39267085 Free PMC article. Clinical Trial.
-
Radiotherapy boost to the primary tumour in locally advanced rectal cancer: Systematic review of practices and meta-analysis.Clin Transl Radiat Oncol. 2025 Jul 13;54:101014. doi: 10.1016/j.ctro.2025.101014. eCollection 2025 Sep. Clin Transl Radiat Oncol. 2025. PMID: 40703667 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical