Early removal of the infrapatellar fat pad/synovium complex beneficially alters the pathogenesis of moderate stage idiopathic knee osteoarthritis in male Dunkin Hartley guinea pigs
- PMID: 36578046
- PMCID: PMC9795160
- DOI: 10.1186/s13075-022-02971-y
Early removal of the infrapatellar fat pad/synovium complex beneficially alters the pathogenesis of moderate stage idiopathic knee osteoarthritis in male Dunkin Hartley guinea pigs
Abstract
Background: The infrapatellar fat pad (IFP) is the largest adipose deposit in the knee; however, its contributions to the homeostasis of this organ remain undefined. To determine the influence of the IFP and its associated synovium (IFP/synovium complex or IFP/SC) on joint health, this study evaluated the progression of osteoarthritis (OA) following excision of this unit in a rodent model of naturally-occurring disease.
Methods: Male Dunkin-Hartley guinea pigs (n=18) received surgical removal of the IFP in one knee at 3 months of age; contralateral knees received sham surgery as matched internal controls. Mobility and gait assessments were performed prior to IFP/SC removal and monthly thereafter. Animals were harvested at 7 months of age. Ten set of these knees were processed for microcomputed tomography (microCT), histopathology, transcript expression analyses, and immunohistochemistry (IHC); 8 sets of knees were dedicated to microCT and biomechanical testing (material properties of knee joints tissues and anterior drawer laxity).
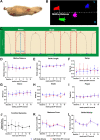
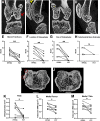
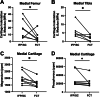
Results: Fibrous connective tissue (FCT) developed in place of the native adipose depot. Gait demonstrated no significant differences between IFP/SC removal and contralateral hindlimbs. MicroCT OA scores were improved in knees containing the FCT. Quantitatively, IFP/SC-containing knees had more osteophyte development and increased trabecular volume bone mineral density (vBMD) in femora and tibiae. Histopathology confirmed maintenance of articular cartilage structure, proteoglycan content, and chondrocyte cellularity in FCT-containing knees. Transcript analyses revealed decreased expression of adipose-related molecules and select inflammatory mediators in FCTs compared to IFP/SCs. This was verified via IHC for two key inflammatory agents. The medial articular cartilage in knees with native IFP/SCs showed an increase in equilibrium modulus, which correlated with increased amounts of magnesium and phosphorus.
Discussion/conclusion: Formation of the FCT resulted in reduced OA-associated changes in both bone and cartilage. This benefit may be associated with: a decrease in inflammatory mediators at transcript and protein levels; and/or improved biomechanical properties. Thus, the IFP/SC may play a role in the pathogenesis of knee OA in this strain, with removal prior to disease onset appearing to have short-term benefits.
Keywords: Biomechanics; Gait; Hartley guinea pig; Inflammation; Infrapatellar fat pad/synovium complex; Osteoarthritis; Trace elements.
© 2022. The Author(s).
Conflict of interest statement
No authors have any conflicts of interest to disclose for this work.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

