Resistant Chronic Spontaneous Urticaria - A Case Series Narrative Review of Treatment Options
- PMID: 36578314
- PMCID: PMC9791268
- DOI: 10.1177/21526575221144951
Resistant Chronic Spontaneous Urticaria - A Case Series Narrative Review of Treatment Options
Abstract
Background: Chronic spontaneous urticaria (CSU) can be extremely debilitating to the patient and challenging for the treating clinician. The National Institute of Health and Clinical Excellence (NICE) in the United Kingdom (UK) recommendation of omalizumab for patients who fail to respond to high-dose anti-histamines has improved treatment options and quality of life. However, there is still lack of clear guidelines for treatment of patients resistant to standard and anti-IgE therapies.
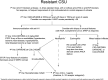
Methods: We discuss the therapeutic strategies employed among nine extremely resistant CSU cases and the heterogeneity between guidelines from different societies.
Results: Patients with anti-histamine-resistant urticaria either remained on omalizumab or started on immunosuppressive drugs (dapsone or ciclosporin) when they stopped responding to omalizumab. We used clinical assessment, skin biopsies (when available) and previous published reports to consider dapsone (for predominantly neutrophilic infiltration), or ciclosporin at doses between 2 and 4 mg/kg/day. One patient with ciclosporin-resistant urticaria responded to mycophenolate mofetil. Two patients remain on long-term omalizumab due to its relative safety and efficacy including 1 patient with underlying antibody deficiency where omalizumab was preferred over risks of using immunosuppressive medications.
Conclusions: These case studies bring to light the real-world difficulties in managing patients with resistant CSU and the need for generating the evidence base on alternative therapeutic options such as synergistic use of biologics and immunosuppressive drugs.
Keywords: Chronic spontaneous urticaria; immunosuppressive drugs; omalizumab; resistant urticaria.
© The Author(s) 2022.
Figures
References
-
- Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63(6):777–780. - PubMed
-
- Weller K, Groffik A, Church MK, et al. Development and validation of the Urticaria control test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol. 2014;133(5):1365–1372. - PubMed
-
- Ohanyan T, Schoepke N, Bolukbasi B, et al. Responsiveness and minimal important difference of the urticaria control test. J Allergy Clin Immunol. 2017;140(6):1710–1713. - PubMed
-
- Zuberbier T, Abdul Latiff AH, Abuzakouk M, et al. The international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77(3):734–766. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

