The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation
- PMID: 36578344
- PMCID: PMC9790997
- DOI: 10.3389/fpls.2022.1044896
The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation
Abstract
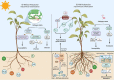
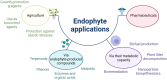
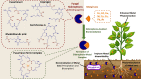
The global environment is dominated by various small exotic substances, known as secondary metabolites, produced by plants and microorganisms. Plants and fungi are particularly plentiful sources of these molecules, whose physiological functions, in many cases, remain a mystery. Fungal secondary metabolites (SM) are a diverse group of substances that exhibit a wide range of chemical properties and generally fall into one of four main family groups: Terpenoids, polyketides, non-ribosomal peptides, or a combination of the latter two. They are incredibly varied in their functions and are often related to the increased fitness of the respective fungus in its environment, often competing with other microbes or interacting with plant species. Several of these metabolites have essential roles in the biological control of plant diseases by various beneficial microorganisms used for crop protection and biofertilization worldwide. Besides direct toxic effects against phytopathogens, natural metabolites can promote root and shoot development and/or disease resistance by activating host systemic defenses. The ability of these microorganisms to synthesize and store biologically active metabolites that are a potent source of novel natural compounds beneficial for agriculture is becoming a top priority for SM fungi research. In this review, we will discuss fungal-plant secondary metabolites with antifungal properties and the role of signaling molecules in induced and acquired systemic resistance activities. Additionally, fungal secondary metabolites mimic plant promotion molecules such as auxins, gibberellins, and abscisic acid, which modulate plant growth under biotic stress. Moreover, we will present a new trend regarding phytoremediation applications using fungal secondary metabolites to achieve sustainable food production and microbial diversity in an eco-friendly environment.
Keywords: Arbuscular mycorrhizal fungi (AMF); biotrophic fungi; phytopathogenic fungi; phytoremediation; plant metabolic response; secondary metabolites; siderophore; soil mycobiota.
Copyright © 2022 Elhamouly, Hewedy, Zaitoon, Miraples, Elshorbagy, Hussien, El-Tahan and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Succession of endophytic fungi and arbuscular mycorrhizal fungi associated with the growth of plant and their correlation with secondary metabolites in the roots of plants.BMC Plant Biol. 2021 Apr 5;21(1):165. doi: 10.1186/s12870-021-02942-6. BMC Plant Biol. 2021. PMID: 33820543 Free PMC article.
-
A review on endophytic fungi: a potent reservoir of bioactive metabolites with special emphasis on blight disease management.Arch Microbiol. 2024 Feb 28;206(3):129. doi: 10.1007/s00203-023-03828-x. Arch Microbiol. 2024. PMID: 38416214 Review.
-
Biofertilizers and sustainable agriculture: exploring arbuscular mycorrhizal fungi.Appl Microbiol Biotechnol. 2017 Jun;101(12):4871-4881. doi: 10.1007/s00253-017-8344-z. Epub 2017 May 25. Appl Microbiol Biotechnol. 2017. PMID: 28547568 Review.
-
Arbuscular mycorrhizal Fungi and Changes in Primary and Secondary Metabolites.Plants (Basel). 2022 Aug 23;11(17):2183. doi: 10.3390/plants11172183. Plants (Basel). 2022. PMID: 36079565 Free PMC article. Review.
-
Secondary metabolites in fungus-plant interactions.Front Plant Sci. 2015 Aug 6;6:573. doi: 10.3389/fpls.2015.00573. eCollection 2015. Front Plant Sci. 2015. PMID: 26300892 Free PMC article. Review.
Cited by
-
Investigating the antioxidant activity enhancer effect of Cyamopsis tetragonoloba seed extract on phenolic phytochemicals.Front Plant Sci. 2023 Mar 8;14:1131173. doi: 10.3389/fpls.2023.1131173. eCollection 2023. Front Plant Sci. 2023. PMID: 36968395 Free PMC article.
-
Strategies for increasing saikosaponins accumulation in Bupleurum: insights from environmental and microbial regulation.Planta. 2025 Jun 23;262(2):35. doi: 10.1007/s00425-025-04748-4. Planta. 2025. PMID: 40549222 Review.
-
Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses.Plants (Basel). 2023 Aug 29;12(17):3102. doi: 10.3390/plants12173102. Plants (Basel). 2023. PMID: 37687353 Free PMC article. Review.
-
Remedying SARS-CoV-2 through nature: a review highlighting the potentiality of herbs, trees, mushrooms, and endophytic microorganisms in controlling Coronavirus.Planta. 2025 Mar 16;261(4):89. doi: 10.1007/s00425-025-04647-8. Planta. 2025. PMID: 40089556 Review.
-
Regulation and induction of fungal secondary metabolites: a comprehensive review.Arch Microbiol. 2025 Jul 1;207(8):189. doi: 10.1007/s00203-025-04386-0. Arch Microbiol. 2025. PMID: 40590991 Review.
References
-
- Akone S. H., Mándi A., Kurtán T., Hartmann R., Lin W., Daletos G., et al. . (2016). Inducing secondary metabolite production by the endophytic fungus chaetomium sp. through fungal–bacterial co-culture and epigenetic modification. Tetrahedron 72 (41), 6340–6347. doi: 10.1016/j.tet.2016.08.022 - DOI
Publication types
LinkOut - more resources
Full Text Sources

