Efficacy and safety of rituximab treatment in patients with idiopathic inflammatory myopathies: A systematic review and meta-analysis
- PMID: 36578492
- PMCID: PMC9791086
- DOI: 10.3389/fimmu.2022.1051609
Efficacy and safety of rituximab treatment in patients with idiopathic inflammatory myopathies: A systematic review and meta-analysis
Abstract
Objective: Idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of autoimmune diseases with various subtypes, myositis-specific antibodies, and affect multiple systems. The treatment of IIMs remains challenging, especially for refractory myositis. In addition to steroids and traditional immunosuppressants, rituximab (RTX), a B cell-depleting monoclonal antibody, is emerging as an alternative treatment for refractory myositis. However, the therapeutic response to RTX remains controversial. This meta-analysis aimed to systematically evaluate the efficacy and safety of RTX in patients with IIMs, excluding sporadic inclusion body myositis.
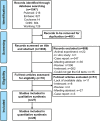
Methods: PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, and WanFang Data were searched for relevant studies. The overall effective rate, complete response rate, and partial response rate were calculated to assess the efficacy of RTX. The incidences of adverse events, infection, severe adverse events, severe infection, and infusion reactions were collected to evaluate the safety of RTX. Subgroup analyses were performed using IIM subtypes, affected organs, continents, and countries. We also performed a sensitivity analysis to identify the sources of heterogeneity.
Results: A total of 26 studies were included in the quantitative analysis, which showed that 65% (95% confidence interval [CI]: 54%, 75%) of patients with IIMs responded to RTX, 45% (95% CI: 22%, 70%) of patients achieved a complete response, and 39% (95% CI: 26%, 53%) achieved a partial response. Subgroup analyses indicated that the overall efficacy rates in patients with refractory IIMs, dermatomyositis and polymyositis, as well as anti-synthetase syndrome were 62%, 68%, and 62%, respectively. The overall efficacy rates for muscle, lungs, and skin involvement were 59%, 65%, and 81%, respectively. In addition, studies conducted in Germany and the United States showed that patients with IIMs had an excellent response to RTX, with an effective rate of 90% and 77%, respectively. The incidence of severe adverse events and infections was 8% and 2%, respectively.
Conclusion: RTX may be an effective and relatively safe treatment choice in patients with IIMs, especially for refractory cases. However, further verification via randomized controlled trials is warranted.
Keywords: efficacy; idiopathic inflammatory myopathies; meta-analysis; rituximab; safety.
Copyright © 2022 Zhen, Hou, Zhao, Ma, Dai and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Lundberg I, Tjärnlund A, Bottai M, Werth V, Pilkington C, Visser M, et al. . 2017 European league against Rheumatism/American college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann rheumatic Dis (2017) 76(12):1955–64. doi: 10.1136/annrheumdis-2017-211468 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

