Basophils from allergy to cancer
- PMID: 36578500
- PMCID: PMC9791102
- DOI: 10.3389/fimmu.2022.1056838
Basophils from allergy to cancer
Abstract
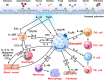
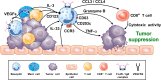
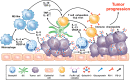
Human basophils, first identified over 140 years ago, account for just 0.5-1% of circulating leukocytes. While this scarcity long hampered basophil studies, innovations during the past 30 years, beginning with their isolation and more recently in the development of mouse models, have markedly advanced our understanding of these cells. Although dissimilarities between human and mouse basophils persist, the overall findings highlight the growing importance of these cells in health and disease. Indeed, studies continue to support basophils as key participants in IgE-mediated reactions, where they infiltrate inflammatory lesions, release pro-inflammatory mediators (histamine, leukotriene C4: LTC4) and regulatory cytokines (IL-4, IL-13) central to the pathogenesis of allergic diseases. Studies now report basophils infiltrating various human cancers where they play diverse roles, either promoting or hampering tumorigenesis. Likewise, this activity bears remarkable similarity to the mounting evidence that basophils facilitate wound healing. In fact, both activities appear linked to the capacity of basophils to secrete IL-4/IL-13, with these cytokines polarizing macrophages toward the M2 phenotype. Basophils also secrete several angiogenic factors (vascular endothelial growth factor: VEGF-A, amphiregulin) consistent with these activities. In this review, we feature these newfound properties with the goal of unraveling the increasing importance of basophils in these diverse pathobiological processes.
Keywords: allergy; angiogenesis; angiopoietins; basophil; cancer; cysteinyl leukotrienes; cytokines; vascular endothelial growth factors.
Copyright © 2022 Poto, Gambardella, Marone, Schroeder, Mattei, Schiavoni and Varricchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ehrlich P. Ueber die specifischen granulationen des blutes. Archiv fuer Anat Und Physiol: Physiol Abteilung (1879) 3:571–9.
-
- Ehrlich P. Beitrage sur theorie und praxis der histologischen farbung. Leipzig, Germany: University of Leipzig; (1878).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

