Apoptosis regulation by the tyrosine-protein kinase CSK
- PMID: 36578781
- PMCID: PMC9792154
- DOI: 10.3389/fcell.2022.1078180
Apoptosis regulation by the tyrosine-protein kinase CSK
Abstract
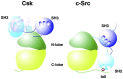
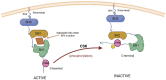
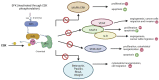
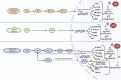
C-terminal Src kinase (CSK) is a cytosolic tyrosine-protein kinase with an important role in regulating critical cellular decisions, such as cellular apoptosis, survival, proliferation, cytoskeletal organization and many others. Current knowledge on the CSK mechanisms of action, regulation and functions is still at an early stage, most of CSK's known actions and functions being mediated by the negative regulation of the SRC family of tyrosine kinases (SFKs) through phosphorylation. As SFKs play a vital role in apoptosis, cell proliferation and survival regulation, SFK inhibition by CSK has a pro-apoptotic effect, which is mediated by the inhibition of cellular signaling cascades controlled by SFKs, such as the MAPK/ERK, STAT3 and PI3K/AKT signaling pathways. Abnormal functioning of CSK and SFK activation can lead to diseases such as cancer, cardiovascular and neurological manifestations. This review describes apoptosis regulation by CSK, CSK inhibition of the SFKs and further explores the clinical relevance of CSK in important pathologies, such as cancer, autoimmune, autoinflammatory, neurologic diseases, hypertension and HIV/AIDS.
Keywords: Csk; MAPK pathway; PI3K-Akt pathway; SFK; Src kinases; apoptosis; src; tyrosine-protein kinase CSK.
Copyright © 2022 Fortner, Chera, Tanca and Bucur.
Figures
References
-
- Alvarado J. J., Tarafdar S., Yeh J. I., Smithgall T. E. (2014). Interaction with the Src homology (SH3-SH2) region of the Src-family kinase Hck structures the HIV-1 Nef dimer for kinase activation and effector recruitment. J. Biol. Chem. 289, 28539–28553. 10.1074/JBC.M114.600031 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

