Efficacy and Safety of Semaglutide for Weight Loss in Obesity Without Diabetes: A Systematic Review and Meta-Analysis
- PMID: 36578889
- PMCID: PMC9758543
- DOI: 10.15605/jafes.037.02.14
Efficacy and Safety of Semaglutide for Weight Loss in Obesity Without Diabetes: A Systematic Review and Meta-Analysis
Abstract
Background: The weight loss benefit of semaglutide in patients with diabetes is well-documented, but its clinical utility in treating obesity among patients without diabetes is less described. We therefore assessed the efficacy and safety of subcutaneous semaglutide as treatment for obesity in patients without diabetes.
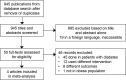
Methodology: A comprehensive search of PubMed/MEDLINE, Cochrane and Google scholar was performed to identify trials on the efficacy and safety of subcutaneous semaglutide on patients with obesity without diabetes. Primary outcome was expressed as percent mean weight difference. Secondary outcomes including risk for gastrointestinal adverse events, discontinuation of treatment and serious adverse events were expressed as risk ratios. These were calculated using the random effects model.
Results: The study included 4 randomized controlled trials having a total of 3,613 individuals with obesity without diabetes. The mean difference for weight reduction was -11.85%, favoring semaglutide [95% confidence interval (CI) (-12.81,-10.90), p<0.00001]. Secondary outcomes showed that the risk of developing gastrointestinal adverse events was 1.59 times more likely with semaglutide (RR 1.59, 95%CI [1.34, 1.88], p<0.00001). Risk for discontinuation due to adverse events was twice as likely in the semaglutide group (RR 2.19, 95%CI [1.36,3.55], p=0.001) and the risk for serious adverse events was 1.6 times more likely for semaglutide (RR1.60, 95%CI [1.24, 2.07], p=0.0003). Serious events were mostly of gastrointestinal and hepatobiliary disorders such as acute pancreatitis and cholelithiasis.
Conclusion: Among individuals with obesity without type 2 diabetes, subcutaneous semaglutide is effective for weight loss with an 11.85% reduction from baseline compared to placebo. This supports the use of semaglutide for weight management in obesity. However, risk of gastrointestinal adverse events, discontinuation of treatment and serious adverse events were higher in the semaglutide group versus placebo.
Keywords: Glucagon-like Peptide -1; obesity; semaglutide; weight loss.
© 2022 Journal of the ASEAN Federation of Endocrine Societies.
Conflict of interest statement
OAD reports receiving consulting fees from Eli Lilly and Novo Nordisk for service outside the submitted work, as well as honoraria for speaking engagements from Astra Zeneca, Novo Nordisk, and Eli Lilly outside the submitted work. HCT and MMM declare no conflict of interest in association with this study.
Figures




References
-
- World Health Organization . Obesity and overweight. Available from: https://www.who.int/news-room/fact-sheets/detail/obesityand-overweight. Accessed 15 May 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
