To sleep or not to sleep: An Italian Control-IQ-uestion
- PMID: 36578959
- PMCID: PMC9790911
- DOI: 10.3389/fendo.2022.996453
To sleep or not to sleep: An Italian Control-IQ-uestion
Abstract
Objective: Tandem Control-IQ is an advanced hybrid closed loop (AHCL) system with a Sleep Activity Mode to intensify glycemic control overnight. The aim of the study is to evaluate the effectiveness of using Sleep Mode or not among Tandem Control-IQ users.
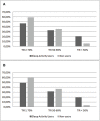
Research design and methods: We performed a retrospective Tandem Control-IQ data download for patients followed at IRCCS G. Gaslini Pediatric Diabetes Centre. We divided the patients into group 1 (Sleep Mode users) and group 2 (non-users) and compared their overall glycemic data, particularly during nighttime.
Results: Group 1 (n = 49) does not show better nocturnal glycemic control as expected when compared with group 2 (n = 34). Group 2 shows a nighttime TIR% of 69.50 versus 66.25 (p = 0.20). Only the patients who do not use Sleep Mode and with sensor and automatic mode use ≥90% reached TIR >70% during nighttime, as well as lower nocturnal TAR% (18.80 versus 21.78, p = 0.05).
Conclusions: This is the first study that evaluates the real-life effectiveness of the use of Sleep Mode in young patients with T1D. Control-IQ Sleep Activity Mode may not be as effective in Italian patients as in American patients due to the different habits.
Keywords: AHCL (Advanced Hybrid Closed Loop); CGM (continuous glucose monitoring); TIR (time in range); Type 1 diabetes (T1D); sleep; tandem control-IQ.
Copyright © 2022 Bassi, Strati, Andreottola, Calevo, d’Annunzio, Maghnie and Minuto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med (1993) 329(14):977–86. doi: 10.1056/NEJM199309303291401 - DOI - PubMed
-
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. . Diabetes control and complications Trial/Epidemiology of diabetes interventions and complications (DCCT/EDIC) study research group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. . N Engl J Med (2005) 353(25):2643–53. doi: 10.1056/NEJMoa052187 - DOI - PMC - PubMed
-
- Cherubini V, Bonfanti R, Casertano A, De Nitto E, Iannilli A, Lombardo F, et al. . Time in range in children with type 1 diabetes using treatment strategies based on nonautomated insulin delivery systems in the real world. Diabetes Technol Ther (2020) 22(7):509–15. doi: 10.1089/dia.2020.0031 - DOI - PubMed
-
- Commissioner O of the. FDA Approves First Automated Insulin Delivery . Device for Type 1 Diabetes. FDA; (2020). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-a.... (Accessed 2021 Aug 17).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical