Integration of a personalised mobile health (mHealth) application into the care of patients with brain tumours: proof-of-concept study (IDEAL stage 1)
- PMID: 36579146
- PMCID: PMC9791405
- DOI: 10.1136/bmjsit-2021-000130
Integration of a personalised mobile health (mHealth) application into the care of patients with brain tumours: proof-of-concept study (IDEAL stage 1)
Abstract
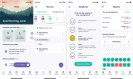
Objectives: Brain tumours lead to significant morbidity including a neurocognitive, physical and psychological burden of disease. The extent to which they impact the multiple domains of health is difficult to capture leading to a significant degree of unmet needs. Mobile health tools such as Vinehealth have the potential to identify and address these needs through real-world data generation and delivery of personalised educational material and therapies. We aimed to establish the feasibility of Vinehealth integration into brain tumour care, its ability to collect real-world and (electronic) patient-recorded outcome (ePRO) data, and subjective improvement in care.
Design: A mixed-methodology IDEAL stage 1 study.
Setting: A single tertiary care centre.
Participants: Six patients consented and four downloaded and engaged with the mHealth application throughout the 12 weeks of the study.
Main outcome measures: Over a 12-week period, we collected real-world and ePRO data via Vinehealth. We assessed qualitative feedback from mixed-methodology surveys and semistructured interviews at recruitment and after 2 weeks.
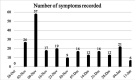
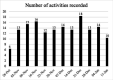
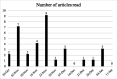
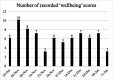
Results: 565 data points were captured including, but not limited to: symptoms, activity, well-being and medication. EORTC QLQ-BN20 and EQ-5D-5L completion rates (54% and 46%) were impacted by technical issues; 100% completion rates were seen when ePROs were received. More brain cancer tumour-specific content was requested. All participants recommended the application and felt it improved care.
Conclusions: Our findings indicate value in an application to holistically support patients living with brain cancer tumours and established the feasibility and safety of further studies to more rigorously assess this.
Keywords: health care quality, access, and evaluation; health technology; patient outcome assessment; process assessment (health care).
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GK, RP and RM are/were all employees of Vinehealth throughout the duration of the study. No other conflicts of interest declared.
Figures
References
-
- Cancer Research UK . Worldwide cancer statistics, 2015. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/w... [Accessed 18 Aug 2020].
Grants and funding
LinkOut - more resources
Full Text Sources
