Impact of Salmonella genome rearrangement on gene expression
- PMID: 36579163
- PMCID: PMC9783417
- DOI: 10.1002/evl3.305
Impact of Salmonella genome rearrangement on gene expression
Abstract
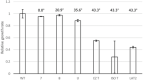
In addition to nucleotide variation, many bacteria also undergo changes at a much larger scale via rearrangement of their genome structure (GS) around long repeat sequences. These rearrangements result in genome fragments shifting position and/or orientation in the genome without necessarily affecting the underlying nucleotide sequence. To date, scalable techniques have not been applied to GS identification, so it remains unclear how extensive this variation is and the extent of its impact upon gene expression. However, the emergence of multiplexed, long-read sequencing overcomes the scale problem, as reads of several thousand bases are routinely produced that can span long repeat sequences to identify the flanking chromosomal DNA, allowing GS identification. Genome rearrangements were generated in Salmonella enterica serovar Typhi through long-term culture at ambient temperature. Colonies with rearrangements were identified via long-range PCR and subjected to long-read nanopore sequencing to confirm genome variation. Four rearrangements were investigated for differential gene expression using transcriptomics. All isolates with changes in genome arrangement relative to the parent strain were accompanied by changes in gene expression. Rearrangements with similar fragment movements demonstrated similar changes in gene expression. The most extreme rearrangement caused a large imbalance between the origin and terminus of replication and was associated with differential gene expression as a factor of distance moved toward or away from the origin of replication. Genome structure variation may provide a mechanism through which bacteria can quickly adapt to new environments and warrants routine assessment alongside traditional nucleotide-level measures of variation.
Keywords: Genome structure; RNAseq; long‐read sequencing.
© 2022 The Authors. Evolution Letters published by Wiley Periodicals LLC on behalf of Society for the Study of Evolution (SSE) and European Society for Evolutionary Biology (ESEB).
Conflict of interest statement
GCL has previously consulted for RevoluGen Ltd on bioinformatic analyses. Fire Monkey DNA extraction kits were provided free of charge by RevoluGen in this project.
Figures