Hyperacute immune responses associate with immediate neuropathology and motor dysfunction in large vessel occlusions
- PMID: 36579400
- PMCID: PMC9930422
- DOI: 10.1002/acn3.51719
Hyperacute immune responses associate with immediate neuropathology and motor dysfunction in large vessel occlusions
Abstract
Objective: Despite successful endovascular therapy, a proportion of stroke patients exhibit long-term functional decline, regardless of the cortical reperfusion. Our objective was to evaluate the early activation of the adaptive immune response and its impact on neurological recovery in patients with large vessel occlusion (LVO).
Methods: Nineteen (13 females, 6 males) patients with acute LVO were enrolled in a single-arm prospective cohort study. During endovascular therapy (EVT), blood samples were collected from pre and post-occlusion, distal femoral artery, and median cubital vein (controls). Cytokines, chemokines, cellular and functional profiles were evaluated with immediate and follow-up clinical and radiographic parameters, including cognitive performance and functional recovery.
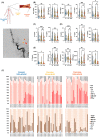
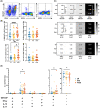
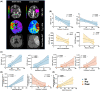
Results: In the hyperacute phase (within hours), adaptive immune activation was observed in the post-occlusion intra-arterial environment (post). Ischemic vascular tissue had a significant increase in T-cell-related cytokines, including IFN-γ and MMP-9, while GM-CSF, IL-17, TNF-α, IL-6, MIP-1a, and MIP-1b were decreased. Cellularity analysis revealed an increase in inflammatory IL-17+ and GM-CSF+ helper T-cells, while natural killer (NK), monocytes and B-cells were decreased. A correlation was observed between hypoperfused tissue, infarct volume, inflammatory helper, and cytotoxic T-cells. Moreover, helper and cytotoxic T-cells were also significantly increased in patients with improved motor function at 3 months.
Interpretation: We provide evidence of the activation of the inflammatory adaptive immune response during the hyperacute phase and the association of pro-inflammatory cytokines with greater ischemic tissue and worsening recovery after successful reperfusion. Further characterization of these immune pathways is warranted to test selective immunomodulators during the early stages of stroke rehabilitation.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors have no financial conflicts of interest relevant to this study.
Figures


References
-
- De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in stroke brain damage. Stroke. 2016;47(4):1165‐1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
