Protective effects of activated vitamin D receptor on radiation-induced intestinal injury
- PMID: 36579449
- PMCID: PMC9843524
- DOI: 10.1111/jcmm.17645
Protective effects of activated vitamin D receptor on radiation-induced intestinal injury
Abstract
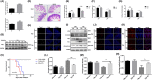
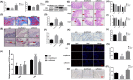
Radiation-induced intestinal injury (RIII) is a common complication after radiation therapy in patients with pelvic, abdominal, or retroperitoneal tumours. Recently, in the model of DSS (Dextran Sulfate Sodium Salt) -induced intestinal inflammatory injury, it has been found in the study that transgenic mice expressing hVDR in IEC (Intestinal Epithelial Cell) manifest highly anti-injury properties in colitis, suggesting that activated VDR in the epithelial cells of intestine may inhibit colitis by protecting the mucosal epithelial barrier. In this study, we investigated the effect of the expression and regulation of VDR on the protection of RIII, and the radiosensitivity in vitro experiments, and explored the initial mechanism of VDR in regulating radiosensitivity of IEC. As a result, we found that the expression of VDR in intestinal tissues and cells in mice can be induced by ionizing radiation. VDR agonists are able to prolong the average survival time of mice after radiation and reduce the radiation-induced intestinal injury. For lack of vitamin D, the radiosensitivity of intestinal epithelial cells in mice increased, which can be reduced by VDR activation. Ensuing VDR activation, the radiation-induced intestinal stem cells damage is decreased, and the regeneration and differentiation of intestinal stem cells is promoted as well. Finally, on the basis of sequencing analysis, we validated and found that VDR may target the HIF/PDK1 pathway to mitigate RIII. We concluded that agonism or upregulation of VDR expression attenuates radiation-induced intestinal damage in mice and promotes the repair of epithelial damage in intestinal stem cells.
Keywords: HIF/PDK1; radiation protection; radiation-induced intestinal injury; tumour radiotherapy; vitamin D.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no potential conflict of interests.
Figures





Similar articles
-
Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis.J Clin Invest. 2013 Sep;123(9):3983-96. doi: 10.1172/JCI65842. Epub 2013 Aug 15. J Clin Invest. 2013. PMID: 23945234 Free PMC article.
-
Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier.Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G208-16. doi: 10.1152/ajpgi.00398.2007. Epub 2007 Oct 25. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 17962355
-
Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis.Nutrients. 2021 Aug 24;13(9):2910. doi: 10.3390/nu13092910. Nutrients. 2021. PMID: 34578802 Free PMC article.
-
Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation.J Steroid Biochem Mol Biol. 2015 Apr;148:179-83. doi: 10.1016/j.jsbmb.2015.01.011. Epub 2015 Jan 17. J Steroid Biochem Mol Biol. 2015. PMID: 25603468 Free PMC article. Review.
-
Radiation-Induced Intestinal Injury: Injury Mechanism and Potential Treatment Strategies.Toxics. 2023 Dec 10;11(12):1011. doi: 10.3390/toxics11121011. Toxics. 2023. PMID: 38133412 Free PMC article. Review.
Cited by
-
The recovery of intestinal barrier function and changes in oral microbiota after radiation therapy injury.Front Cell Infect Microbiol. 2024 Feb 7;13:1288666. doi: 10.3389/fcimb.2023.1288666. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38384432 Free PMC article.
-
Promising roles of vitamin D receptor and APRO family proteins for the development of cancer stem cells targeted malignant tumor therapy.Oncol Res. 2025 Apr 18;33(5):1007-1017. doi: 10.32604/or.2025.059657. eCollection 2025. Oncol Res. 2025. PMID: 40296902 Free PMC article. Review.
-
Influence of Vitamin D Receptor Signalling and Vitamin D on Colonic Epithelial Cell Fate Decisions in Ulcerative Colitis.J Crohns Colitis. 2024 Oct 15;18(10):1672-1689. doi: 10.1093/ecco-jcc/jjae074. J Crohns Colitis. 2024. PMID: 38747639 Free PMC article.
-
Nuclear receptors as novel regulators that modulate cancer radiosensitivity and normal tissue radiotoxicity.Mol Cancer. 2025 May 30;24(1):155. doi: 10.1186/s12943-025-02362-2. Mol Cancer. 2025. PMID: 40442680 Free PMC article. Review.
-
Itaconate: A Potent Macrophage Immunomodulator.Inflammation. 2023 Aug;46(4):1177-1191. doi: 10.1007/s10753-023-01819-0. Epub 2023 May 4. Inflammation. 2023. PMID: 37142886 Free PMC article. Review.
References
-
- Lu L, Li W, Chen L, et al. Radiation‐induced intestinal damage: latest molecular and clinical developments. Future Oncol. 2019;15(35):4105‐4118. - PubMed
-
- Nussbaum ML, Campana TJ, Weese JL. Radiation‐induced intestinal injury. Clin Plast Surg. 1993;20(3):573‐580. - PubMed
-
- An N, Liu T, Zhu B, et al. A bidirectional effect of Rac1 inhibition‐protects radiation‐induced intestinal injury while inhibits tumor. Life Sci. 2020;240:117105. - PubMed
-
- Xie LW, Cai S, Zhao TS, Li M, Tian Y. Green tea derivative (−)‐epigallocatechin‐3‐gallate (EGCG) confers protection against ionizing radiation‐induced intestinal epithelial cell death both in vitro and in vivo. Free Radic Biol Med. 2020;161:175‐186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

