Immune checkpoint inhibitors in kidney transplantation
- PMID: 36579684
- PMCID: PMC9811500
- DOI: 10.1097/MOT.0000000000001036
Immune checkpoint inhibitors in kidney transplantation
Abstract
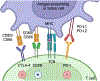
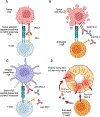
Purpose of review: The development of immune checkpoint inhibitor (ICI) immunotherapy has revolutionized the treatment of several cancers. Malignancies are one of the leading causes of death in solid organ transplant recipients (SOTRs). Although ICI treatment may be an effective option in treating malignancies in SOTRs, concerns about triggering allograft rejection have been raised in this population. Herein, we will review currently available data regarding patients, allograft and malignancy outcomes in SOTRs who received ICI therapy.
Recent findings: Cancer incidence is three to five-fold higher among SOTRs, compared with the general population. Skin cancer is the most prevalent cancer after transplant, followed by kidney cancer, lymphoma and Kaposi sarcoma. There are no large prospective studies evaluating ICI therapy's use for treating cancers in SOTRs. However, retrospective studies have shown that ICI treatment may be associated with improved malignancy outcomes and overall survival (OS). However, the risk of allograft rejection is high (around 40%) of whom about half lose their allograft. Maintaining higher levels of immunosuppression may be associated with a lower risk of allograft rejection, but potentially worse malignancy outcomes.
Summary: Although ICI treatment may be associated with improved patient and malignancy outcomes, the risk of allograft rejection and loss are high. Prospective studies are needed to confirm the benefits of ICI therapy in SOTRs and to evaluate the optimal immunosuppression regimen modifications, if any, to improve patient, malignancy and allograft outcomes in transplant recipients.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Figures
Similar articles
-
Immune Checkpoint Inhibitors in Solid Organ Transplant Recipients With Advanced Skin Cancers-Emerging Strategies for Clinical Management.Transplantation. 2023 Jul 1;107(7):1452-1462. doi: 10.1097/TP.0000000000004459. Epub 2023 Jun 20. Transplantation. 2023. PMID: 36706163
-
Immune checkpoint inhibitor therapy in solid organ transplant recipients: A patient-centered systematic review.J Am Acad Dermatol. 2020 Jun;82(6):1490-1500. doi: 10.1016/j.jaad.2019.07.005. Epub 2019 Jul 11. J Am Acad Dermatol. 2020. PMID: 31302190
-
Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: A single-center analysis.Cancer. 2020 Nov 1;126(21):4780-4787. doi: 10.1002/cncr.33134. Epub 2020 Aug 12. Cancer. 2020. PMID: 32786022 Free PMC article.
-
Cutaneous squamous cell carcinoma in solid organ transplant recipients: Current therapeutic and screening strategies.Transplant Rev (Orlando). 2024 Dec;38(4):100882. doi: 10.1016/j.trre.2024.100882. Epub 2024 Sep 25. Transplant Rev (Orlando). 2024. PMID: 39348772 Review.
-
A Pilot Study of Checkpoint Inhibitors in Solid Organ Transplant Recipients with Metastatic Cutaneous Squamous Cell Carcinoma.Oncologist. 2021 Feb;26(2):133-138. doi: 10.1002/onco.13539. Epub 2020 Oct 15. Oncologist. 2021. PMID: 32969143 Free PMC article.
Cited by
-
[Urogenital tumors following kidney transplantation-monocentric analysis of incidences and overview of urological preventive measures].Urologie. 2024 Apr;63(4):341-350. doi: 10.1007/s00120-024-02317-3. Epub 2024 Mar 21. Urologie. 2024. PMID: 38512472 Free PMC article. German.
-
Use of immune checkpoint inhibitors in solid organ transplant recipients with advanced cutaneous malignancies.Front Transplant. 2023 Oct 30;2:1284740. doi: 10.3389/frtra.2023.1284740. eCollection 2023. Front Transplant. 2023. PMID: 38993910 Free PMC article.
-
Acute Allograft Rejection in Kidney Transplant Recipients Treated With Immune Checkpoint Inhibitors: An Educational Case Report.Can J Kidney Health Dis. 2024 Oct 21;11:20543581241289191. doi: 10.1177/20543581241289191. eCollection 2024. Can J Kidney Health Dis. 2024. PMID: 39444717 Free PMC article.
-
Combined radiation- and immune checkpoint-inhibitor-induced pneumonitis - The challenge to predict and detect overlapping immune-related adverse effects from evolving laboratory biomarkers and clinical imaging.Neoplasia. 2023 May;39:100892. doi: 10.1016/j.neo.2023.100892. Epub 2023 Apr 1. Neoplasia. 2023. PMID: 37011458 Free PMC article.
References
-
- Vajdic CM, McDonald SP, McCredie MRE, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA [Internet]. 2006. Dec 20;296(23):2823–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17179459 - PubMed
-
- Wong G, Chapman JR. Cancers after renal transplantation. Transplant Rev (Orlando). 2008. Apr;22(2):141–9. - PubMed
-
- Howard RJ, Patton PR, Reed AI, Hemming AW, Van Der Werf WJ, Pfaff WW, et al. The changing causes of graft loss and death after kidney transplantation. Transplantation. 2002. Jun;73(12):1923–8. - PubMed
-
- Portuguese AJ, Tykodi SS, Blosser CD, Gooley TA, Thompson JA, Hall ET. Immune Checkpoint Inhibitor Use in Solid Organ Transplant Recipients: A Systematic Review. JNCCN J Natl Compr Cancer Netw. 2022;20(4):406–16. - PubMed
-
It is one of the most comprehensive review paper based upon 119 reported cases of ICI treatment of SOTRs. The paper categorized the cases on the basis of ICI agents, cancer types, immunosuppressive regimen types, and patient variables, It gives a good idea about the risk factors involved in the ICI treatment of SOTRs.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

