Adolescent functional network connectivity prospectively predicts adult anxiety symptoms related to perceived COVID-19 economic adversity
- PMID: 36579796
- PMCID: PMC9880614
- DOI: 10.1111/jcpp.13749
Adolescent functional network connectivity prospectively predicts adult anxiety symptoms related to perceived COVID-19 economic adversity
Abstract
Background: Stressful events, such as the COVID-19 pandemic, are major contributors to anxiety and depression, but only a subset of individuals develop psychopathology. In a population-based sample (N = 174) with a high representation of marginalized individuals, this study examined adolescent functional network connectivity as a marker of susceptibility to anxiety and depression in the context of adverse experiences.
Methods: Data-driven network-based subgroups were identified using an unsupervised community detection algorithm within functional neural connectivity. Neuroimaging data collected during emotion processing (age 15) were extracted from a priori regions of interest linked to anxiety and depression. Symptoms were self-reported at ages 15, 17, and 21 (during COVID-19). During COVID-19, participants reported on pandemic-related economic adversity. Differences across subgroup networks were first examined, then subgroup membership and subgroup-adversity interaction were tested to predict change in symptoms over time.
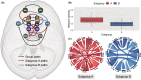
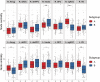
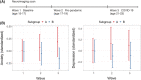
Results: Two subgroups were identified: Subgroup A, characterized by relatively greater neural network variation (i.e., heterogeneity) and density with more connections involving the amygdala, subgenual cingulate, and ventral striatum; and the more homogenous Subgroup B, with more connections involving the insula and dorsal anterior cingulate. Accounting for initial symptoms, subgroup A individuals had greater increases in symptoms across time (β = .138, p = .042), and this result remained after adjusting for additional covariates (β = .194, p = .023). Furthermore, there was a subgroup-adversity interaction: compared with Subgroup B, Subgroup A reported greater anxiety during the pandemic in response to reported economic adversity (β = .307, p = .006), and this remained after accounting for initial symptoms and many covariates (β = .237, p = .021).
Conclusions: A subgrouping algorithm identified young adults who were susceptible to adversity using their personalized functional network profiles derived from a priori brain regions. These results highlight potential prospective neural signatures involving heterogeneous emotion networks that predict individuals at the greatest risk for anxiety when experiencing adverse events.
Keywords: Stress susceptibility; anxiety; functional connectivity; person-specific network.
© 2022 Association for Child and Adolescent Mental Health.
Figures

References
-
- Beck, A.T. , & Clark, D.A. (1988). Anxiety and depression: An information processing perspective. Anxiety Research, 1, 23–36.
-
- Beltz, A. , Dotterer, H. , & Goetschius, L. (2019). GIMME preprocessing: Initial release (version v1. 0). Zenodo . doi: 10.5281/zenodo.2692522 - DOI
-
- Boyce, W.T. , & Ellis, B.J. (2005). Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17, 271–301. - PubMed

